Hey everyone! Ever heard of 21 CFR Part 11 and Good Documentation Practices (GDP)? Sounds kinda... technical, right? Like something locked away in a sterile lab, only to be whispered about by serious scientists. But trust me, it's way more interesting (and useful!) than it sounds. Think of it like this: it's the secret sauce to ensuring that medicines, medical devices, and all sorts of regulated products are safe and effective for you and me.
What's the 411 on 21 CFR Part 11 & Good Documentation Practices?
Okay, let's break it down. 21 CFR Part 11 is a regulation from the U.S. Food and Drug Administration (FDA). It essentially defines the requirements for electronic records and electronic signatures. Imagine trying to verify the safety of a drug based on data stored on a floppy disk from 1985. Yikes! 21 CFR Part 11 helps prevent that kind of nightmare scenario by setting standards for how companies manage their data electronically.
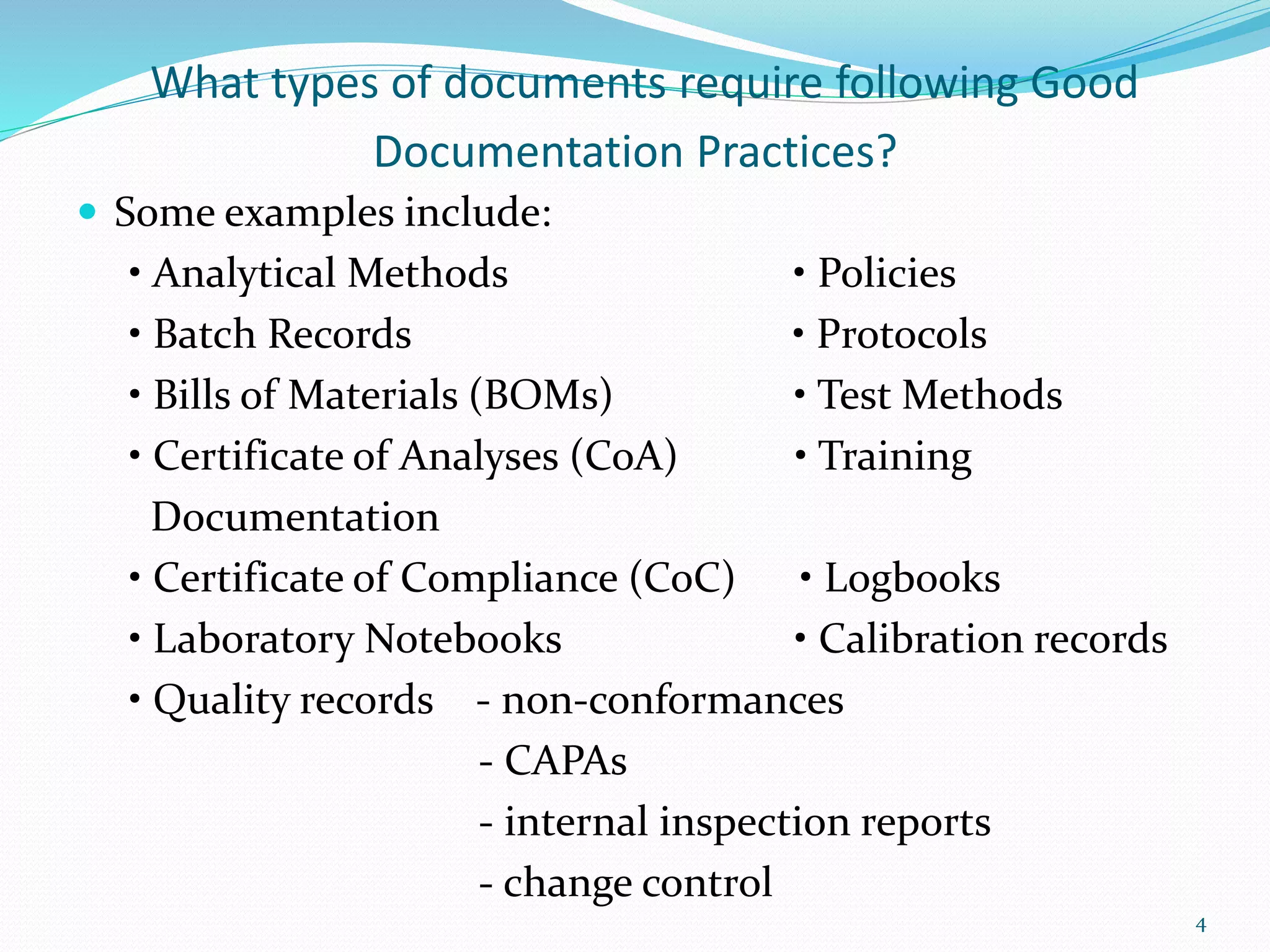
Now, Good Documentation Practices (GDP) are the principles and guidelines that dictate how we create, maintain, and control records (both paper and electronic) throughout a regulated process. GDP is all about making sure that documentation is accurate, complete, consistent, and attributable. Think of it as the foundation upon which reliable and trustworthy data is built.
But why should you care? I mean, unless you're a pharmaceutical executive or a regulatory affairs specialist, what does this have to do with your daily life? Well, consider this: every time you take a medication, use a medical device, or consume a product regulated by the FDA, you're indirectly relying on the fact that someone, somewhere, followed these rules. GDP helps ensure that the products you use are safe, effective, and manufactured to the highest standards. Pretty important, right?
GDP: The Pillars of Truth
So, what makes up good documentation? It's not just about writing things down. It's about writing things down *correctly*. Here are some of the key principles:
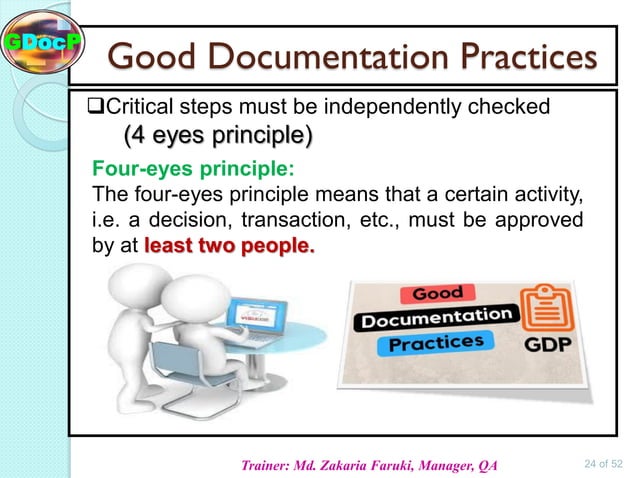
- Attributable: Who created the record? Who modified it? We need to know who did what and when. Think of it like signing your name to a contract.
- Legible: Can you actually read it? This seems obvious, but you'd be surprised! Illegible records are essentially useless. Imagine trying to decipher a doctor's prescription written in ancient hieroglyphics.
- Contemporaneous: Record things as they happen, not days later. Don't rely on your memory – write it down immediately. It's like taking notes during a meeting versus trying to recreate it from memory a week later.
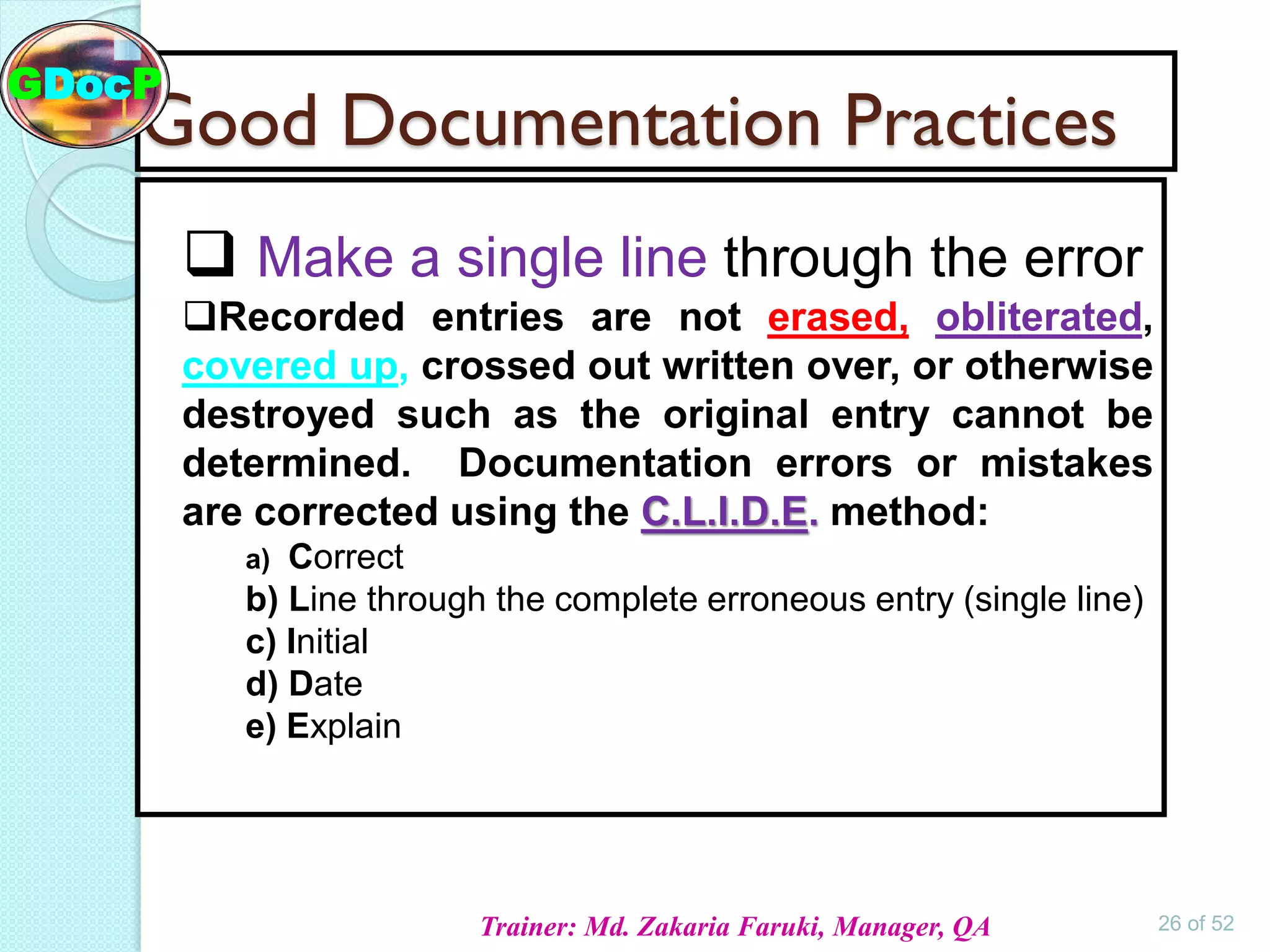
- Original: Use the original document or a certified copy. No photocopies of photocopies of photocopies! We need to be able to trace the information back to its source.
- Accurate: This one's crucial. Make sure the information is correct and reflects what actually happened. No fudging the numbers!
- Complete: Don't leave anything out. A complete record tells the whole story, not just parts of it. Think of it like a recipe – you need all the ingredients and instructions to bake the cake properly.
- Consistent: Data should be consistent across all records. Numbers should add up, dates should match, and terminology should be uniform. Imagine reading a novel where the main character's name changes halfway through – confusing, right?
- Enduring: Records should be stored securely and preserved for the required retention period. Think of it like archiving important historical documents – we need to be able to access them in the future.
- Available: Records should be readily accessible when needed for review, audit, or investigation. No hiding the evidence!
Think of it like building a house. If the foundation (documentation) is weak, the entire structure (the product) is at risk. Good Documentation Practices are the blueprint for a solid, reliable foundation.
Why All the Fuss? What are the Benefits?
So, why all this attention to detail? What's the payoff for all this extra effort? The benefits are numerous, both for companies and for consumers.
- Improved Product Quality: By ensuring that processes are well-documented and controlled, companies can minimize errors and improve the quality of their products.
- Enhanced Regulatory Compliance: Adhering to GDP helps companies comply with FDA regulations and avoid costly penalties.
- Reduced Risk: Proper documentation helps identify and mitigate risks associated with manufacturing processes.
- Faster Problem Solving: When issues arise, good documentation allows companies to quickly identify the root cause and implement corrective actions.
- Increased Efficiency: Well-documented processes are easier to understand and follow, leading to improved efficiency and productivity.
- Greater Trust and Confidence: Consumers have greater trust and confidence in products that are manufactured according to high standards of quality and documentation.
Imagine trying to diagnose a car problem without any service records. You'd be guessing in the dark! Good documentation is like having a detailed service history for every product, allowing companies to quickly identify and resolve any issues that may arise.
Finding that Elusive PDF: Your Guide to 21 CFR Good Documentation Practices
Now, if you're really keen to dive deep into the nitty-gritty details, you might be looking for a 21 CFR Good Documentation Practices PDF. While there isn't one single, official document labeled *exactly* that, the core principles are woven throughout various guidance documents and interpretations of 21 CFR Part 11 and related regulations.
Here's where to look:
- FDA Website: The FDA website (fda.gov) is your best friend. Search for "21 CFR Part 11," "Good Documentation Practices," or "Data Integrity."
- FDA Guidance Documents: The FDA publishes numerous guidance documents that provide detailed information on specific aspects of documentation and data integrity. Look for documents related to pharmaceutical manufacturing, medical devices, and laboratory practices.
- Industry Associations: Organizations like ISPE (International Society for Pharmaceutical Engineering) and PDA (Parenteral Drug Association) offer resources and training on Good Documentation Practices.
- Consultants: If you need expert help, consider consulting with a regulatory affairs specialist or a GMP consultant.
Remember, GDP isn't just about following a checklist. It's about fostering a culture of quality and integrity within your organization. It's about making sure that everyone understands the importance of accurate and reliable documentation. It's about building trust with consumers and regulators alike.
GDP in Everyday Life (Sort Of!)
Okay, so maybe you're not filling out batch records or validating software systems. But the principles of Good Documentation Practices can actually be applied to many aspects of your daily life (in a slightly less formal way, of course!).
- Taking Meeting Minutes: Accurately recording what was discussed and agreed upon during a meeting.
- Keeping a Budget: Tracking your income and expenses to ensure that your finances are in order.
- Following a Recipe: Carefully following the instructions to ensure that your dish turns out as expected.
- Writing a To-Do List: Keeping track of your tasks and deadlines to stay organized and productive.
- Documenting a Project: If you're working on a DIY project, document the steps you took and the materials you used in case you need to troubleshoot later.
Okay, maybe it's a stretch, but the core idea is the same: accurate, complete, and reliable information is always valuable. It's about accountability, traceability, and ensuring that you can learn from your experiences.
Final Thoughts: Documenting for a Better Tomorrow
So, there you have it! Good Documentation Practices may seem like a dry and technical topic, but they are essential for ensuring the safety and effectiveness of the products we use every day. It's about building a foundation of trust and reliability, one record at a time.
Next time you're taking a medication or using a medical device, remember that someone, somewhere, followed these principles to make sure that product is safe and effective for you. And that's pretty cool, don't you think?