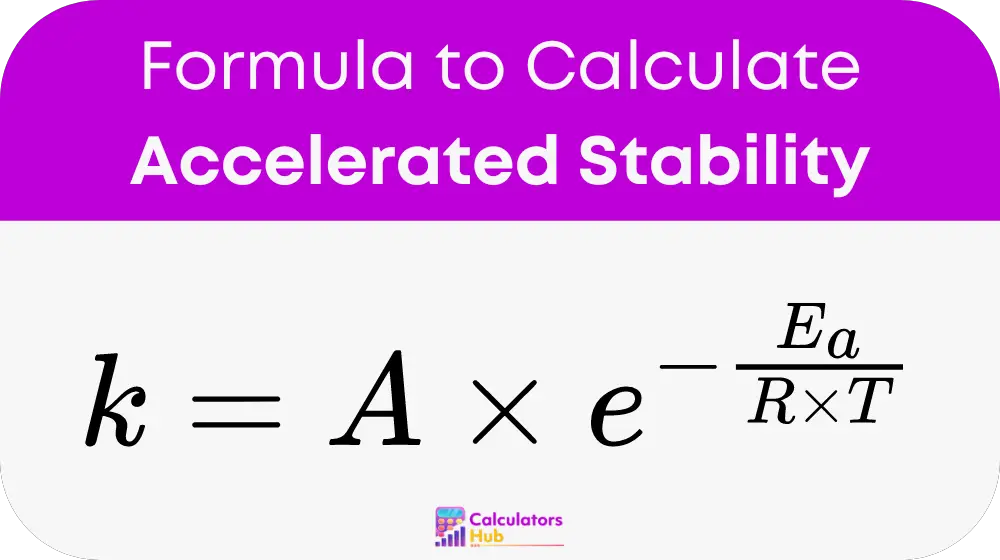Understanding the concept of accelerated stability testing is crucial in various industries, particularly pharmaceuticals, food science, and cosmetics. The core principle revolves around predicting the long-term stability of a product by subjecting it to stressed conditions for a shorter duration. A common point of discussion is the correlation between 3 months of accelerated stability testing and its equivalent in real-time, normal storage conditions.
Accelerated Stability Testing: An Overview
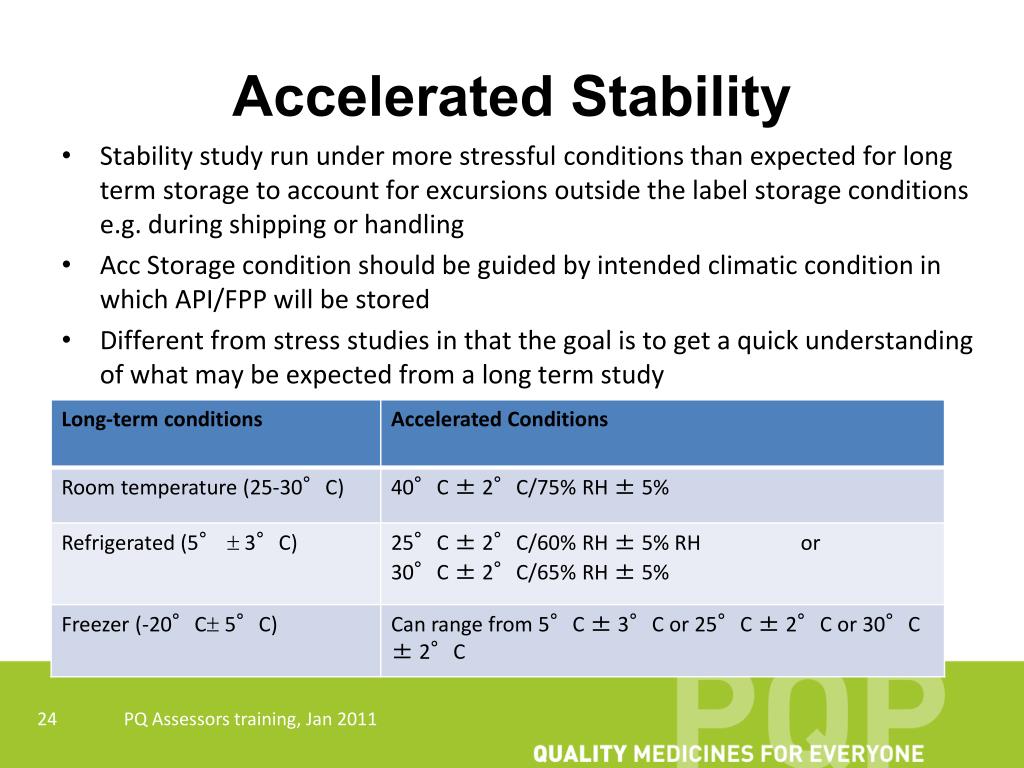
Accelerated stability testing is designed to speed up the degradation processes that a product might undergo during its shelf life. This is achieved by exposing the product to higher temperatures, humidity levels, and sometimes light. The data gathered from these tests helps manufacturers estimate how long a product will remain safe and effective under recommended storage conditions.
Purpose of Accelerated Stability Testing
The primary aims of accelerated stability testing are to:
- Predict shelf life: Determine how long a product can be stored before it degrades beyond acceptable limits.
- Identify degradation pathways: Understand the mechanisms by which the product breaks down, allowing for formulation improvements.
- Assess packaging suitability: Ensure that the packaging protects the product from environmental factors.
- Support regulatory submissions: Provide data required by regulatory agencies for product approval.
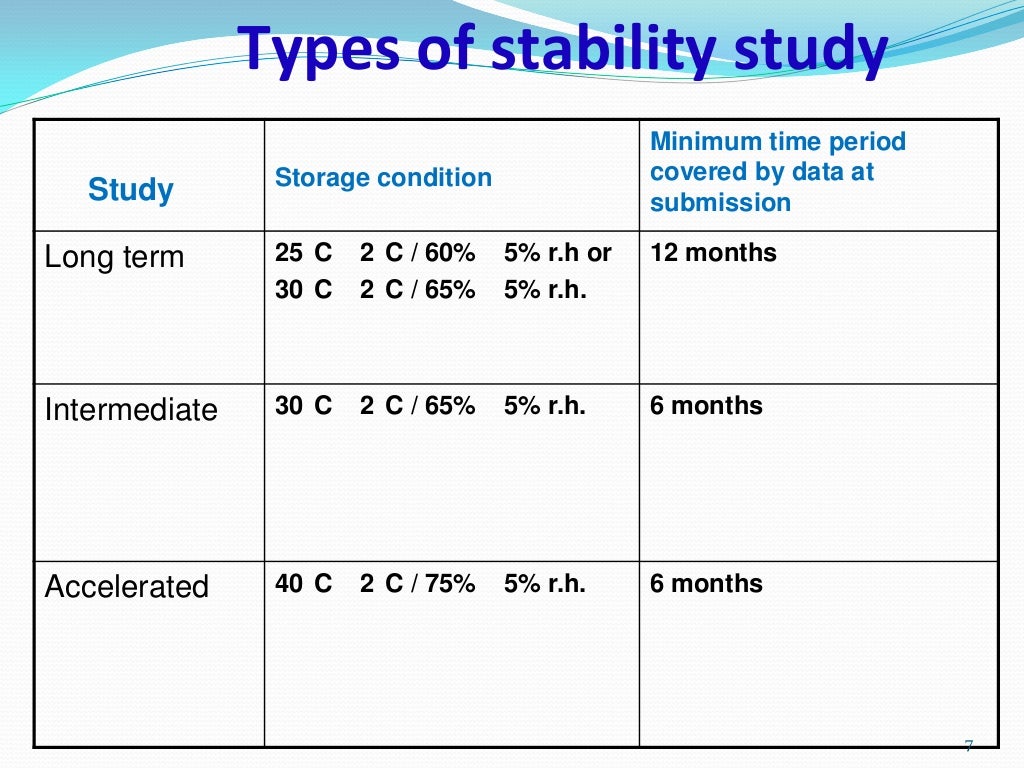
The Equivalence Principle: 3 Months Accelerated
The "3 months accelerated stability equivalent to" question is complex and highly dependent on the specific product and the conditions used during the accelerated testing. There is no universal formula that applies to all scenarios. The equivalence is determined based on the observed degradation rate under accelerated conditions and then extrapolated to predict degradation at normal storage conditions.
Factors Affecting Equivalence
Several factors influence the relationship between accelerated and real-time stability:
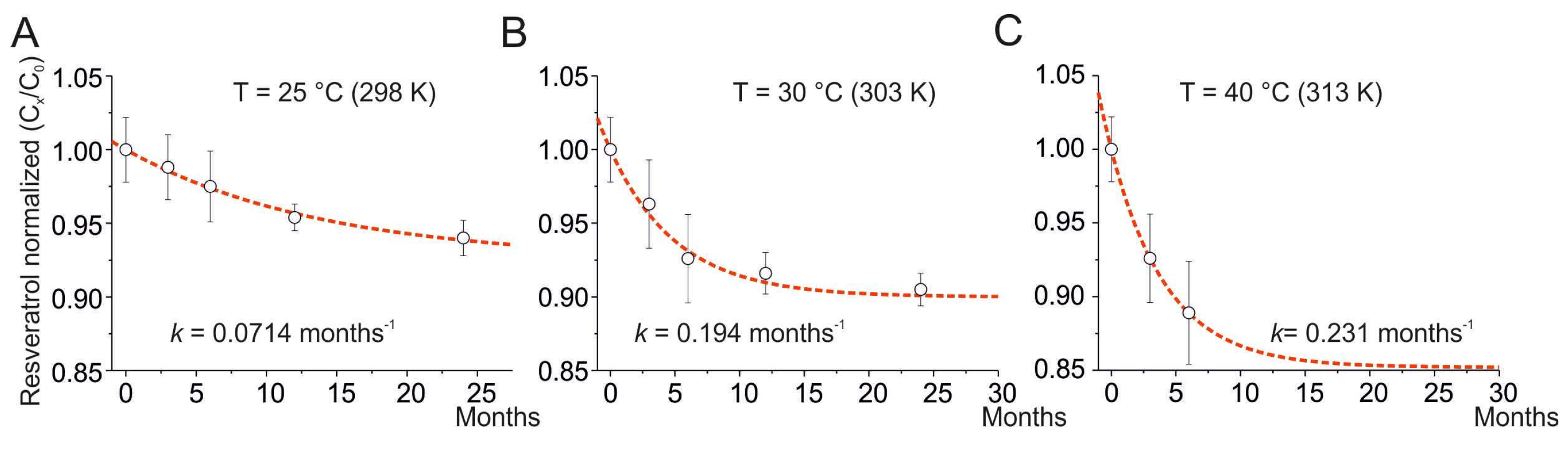
- Temperature: Increased temperature generally accelerates chemical reactions, including degradation. The Arrhenius equation is often used to model the relationship between temperature and reaction rate.
- Humidity: High humidity can promote hydrolysis, oxidation, and microbial growth, all of which can degrade a product.
- Light: Exposure to light, particularly UV light, can cause photodegradation of certain compounds.
- Product Formulation: The composition of the product significantly impacts its stability. Some ingredients are more susceptible to degradation than others.
- Packaging: The type of packaging used can protect the product from environmental factors, thereby affecting its stability.
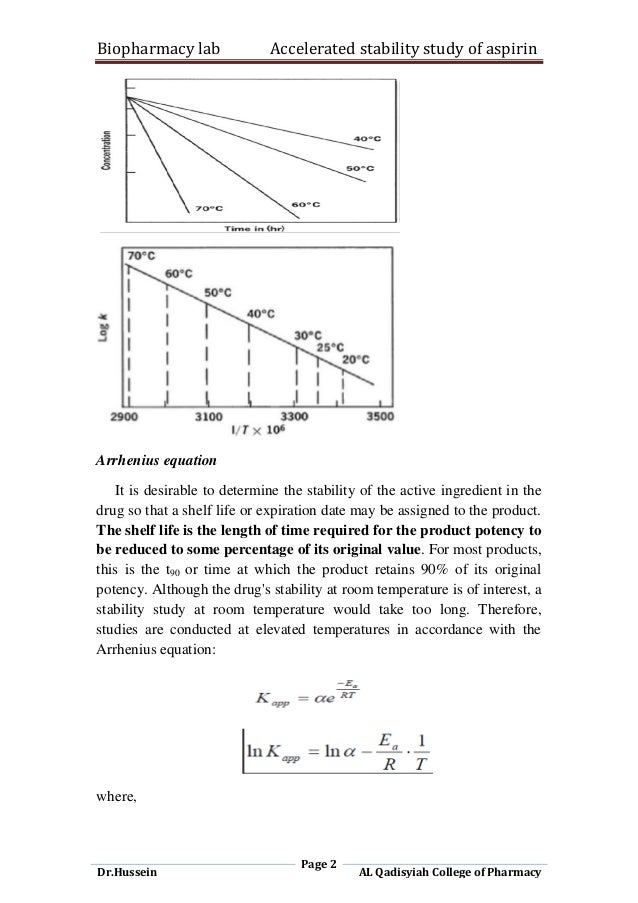
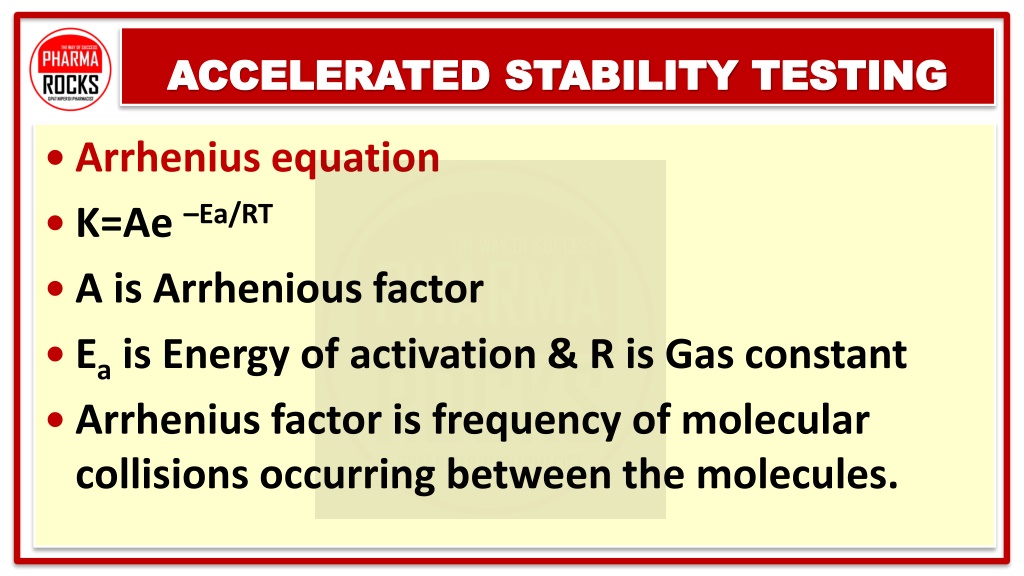
The Arrhenius Equation and Q10 Rule
The Arrhenius equation provides a mathematical framework for understanding the effect of temperature on reaction rates:
k = A * exp(-Ea/RT)
Where:
- k is the rate constant
- A is the pre-exponential factor
- Ea is the activation energy
- R is the gas constant
- T is the absolute temperature
While the Arrhenius equation provides a strong theoretical basis, a simplified approach known as the Q10 rule is often used as a rule of thumb. The Q10 rule states that for every 10°C increase in temperature, the reaction rate doubles or triples. This can be expressed as:
Rate2 = Rate1 * Q10((T2 - T1)/10)
Where:
- Rate1 is the reaction rate at temperature T1
- Rate2 is the reaction rate at temperature T2
- Q10 is the temperature coefficient (typically 2 or 3)
For instance, if a product is stored at 40°C during accelerated testing and its recommended storage temperature is 25°C, and if the Q10 value is assumed to be 3, then the degradation rate at 40°C would be approximately 9 times faster than at 25°C. Therefore, 3 months at 40°C might be roughly equivalent to 27 months (2.25 years) at 25°C.
Specific Examples and Considerations
Let's consider a few hypothetical examples to illustrate the complexity:
Pharmaceutical Product
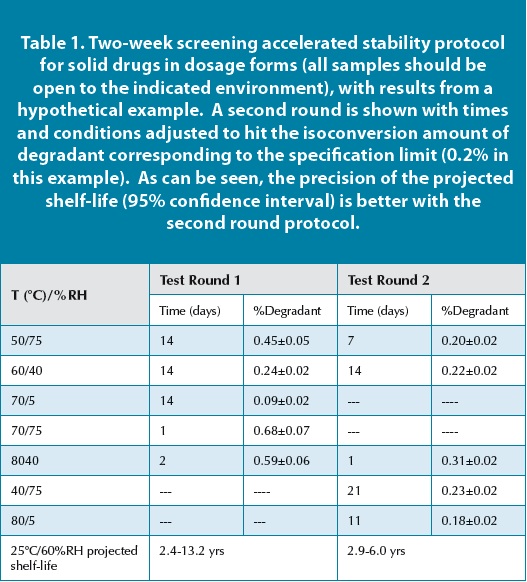
A pharmaceutical product is subjected to accelerated stability testing at 40°C and 75% relative humidity (RH) for 3 months. Assume its recommended storage condition is 25°C and 60% RH. The active pharmaceutical ingredient (API) degrades by 5% during the 3 months of accelerated testing. Using the Arrhenius equation (or a validated Q10 approach based on the specific API's degradation kinetics), and considering the effect of humidity (which might require separate modeling or empirical data), scientists might estimate that this 5% degradation would take approximately 24 months at 25°C and 60% RH. Therefore, in this specific case, 3 months at 40°C/75% RH could be considered equivalent to 2 years at 25°C/60% RH. However, this is a simplified scenario, and a thorough analysis would require much more data points and sophisticated modeling.
Food Product
A food product is tested at 30°C for 3 months. Its intended storage is at refrigerated conditions (4°C). Microbial growth is a primary concern. The product shows a specific level of microbial load at the end of 3 months at 30°C. Extrapolating this data to 4°C requires understanding the microbial growth kinetics at different temperatures. A simple Q10 approximation might be inaccurate because microbial growth often has a more complex relationship with temperature, involving minimum, optimum, and maximum growth temperatures. Therefore, it's often necessary to perform additional stability studies at intermediate temperatures to refine the prediction. In this scenario, 3 months at 30°C might only be equivalent to a few months at 4°C in terms of microbial stability, but could indicate much longer shelf life regarding sensory qualities.
Cosmetic Product
A cosmetic lotion is subjected to 45°C for 3 months. Changes in viscosity, color, and odor are monitored. The formulation is oil-in-water emulsion, and phase separation is a key stability concern. If the lotion shows signs of phase separation after 3 months at 45°C, it suggests that the emulsion is unstable. However, determining the equivalent time at room temperature (e.g., 25°C) is challenging. Emulsion stability is often influenced by factors beyond simple chemical kinetics, such as interfacial tension and droplet size distribution. Therefore, the correlation might be non-linear, and 3 months at 45°C could potentially indicate a shelf life of less than a year at 25°C, depending on the specific formulation and the severity of the phase separation.
Limitations and Caveats
It's important to acknowledge the limitations of accelerated stability testing and the associated equivalence estimations:
- Extrapolation Errors: Extrapolating data from accelerated conditions to normal storage conditions always involves a degree of uncertainty. The further the extrapolation, the greater the potential for error.
- Changes in Degradation Mechanism: At elevated temperatures or humidity levels, the degradation mechanism might change, rendering the accelerated data irrelevant to normal storage conditions.
- Oversimplification: The Arrhenius equation and Q10 rule are simplifications of complex chemical and physical processes. They may not accurately reflect the behavior of all products.
- Packaging Interactions: Accelerated testing can sometimes exacerbate interactions between the product and its packaging, which might not be representative of real-time storage.
Best Practices for Stability Testing
To ensure reliable stability data and accurate predictions, consider the following best practices:
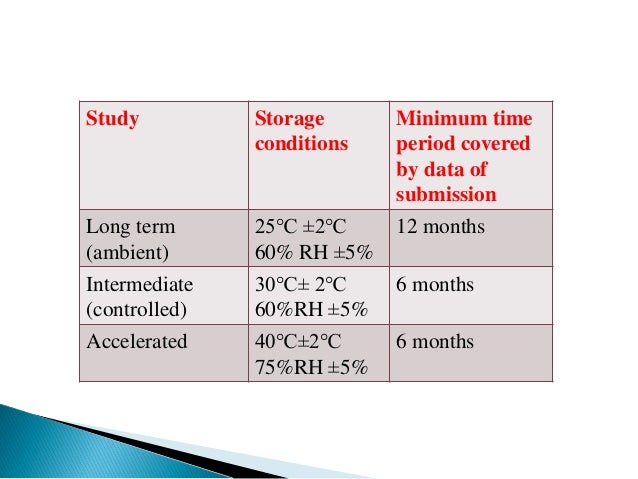
- Proper Study Design: Design the stability study based on established guidelines (e.g., ICH guidelines for pharmaceuticals).
- Appropriate Storage Conditions: Select storage conditions that are relevant to the product and its intended use.
- Relevant Testing Parameters: Monitor parameters that are indicative of product degradation (e.g., assay, degradation products, physical appearance, microbial load).
- Sufficient Time Points: Collect data at multiple time points to establish a clear degradation trend.
- Statistical Analysis: Use appropriate statistical methods to analyze the data and estimate shelf life.
- Real-Time Stability Studies: Supplement accelerated stability data with real-time stability studies to confirm the predictions.
Conclusion
The phrase "3 months accelerated stability equivalent to" is not a fixed value. The actual equivalent time under normal storage conditions varies significantly depending on the product, its formulation, the packaging, and the specific conditions used during the accelerated testing. While the Arrhenius equation and Q10 rule provide useful approximations, they should be applied with caution and validated with empirical data. A thorough understanding of the product's degradation pathways and careful study design are essential for making accurate shelf-life predictions. Understanding these complexities allows for safer, more effective products to reach consumers and reduces potential waste and financial loss.Accelerated stability testing remains a vital tool in product development, ensuring that products maintain their quality and integrity throughout their intended shelf life.