Organic chemistry reactions often involve the transformation of one molecule into another, and predicting the major product of a given reaction is a fundamental skill. This article will guide you through the process of determining the major product in a hypothetical organic reaction. We'll break down the reaction step-by-step, focusing on the key elements that influence the outcome.
Identifying the Reactants and Reagents
The first step is to carefully identify all the reactants and reagents involved in the reaction. This includes the organic starting material, any catalysts, solvents, and other chemicals used. Understanding the structure and properties of each component is crucial for predicting the reaction mechanism and, ultimately, the major product.
Let's assume our reaction involves the following:
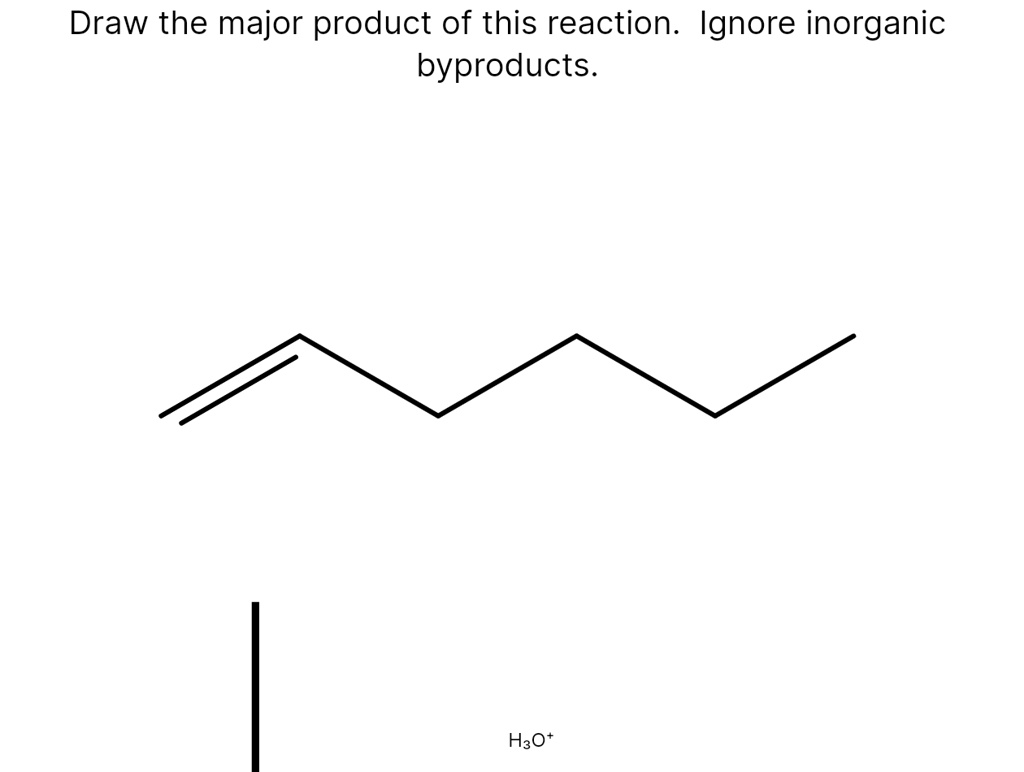
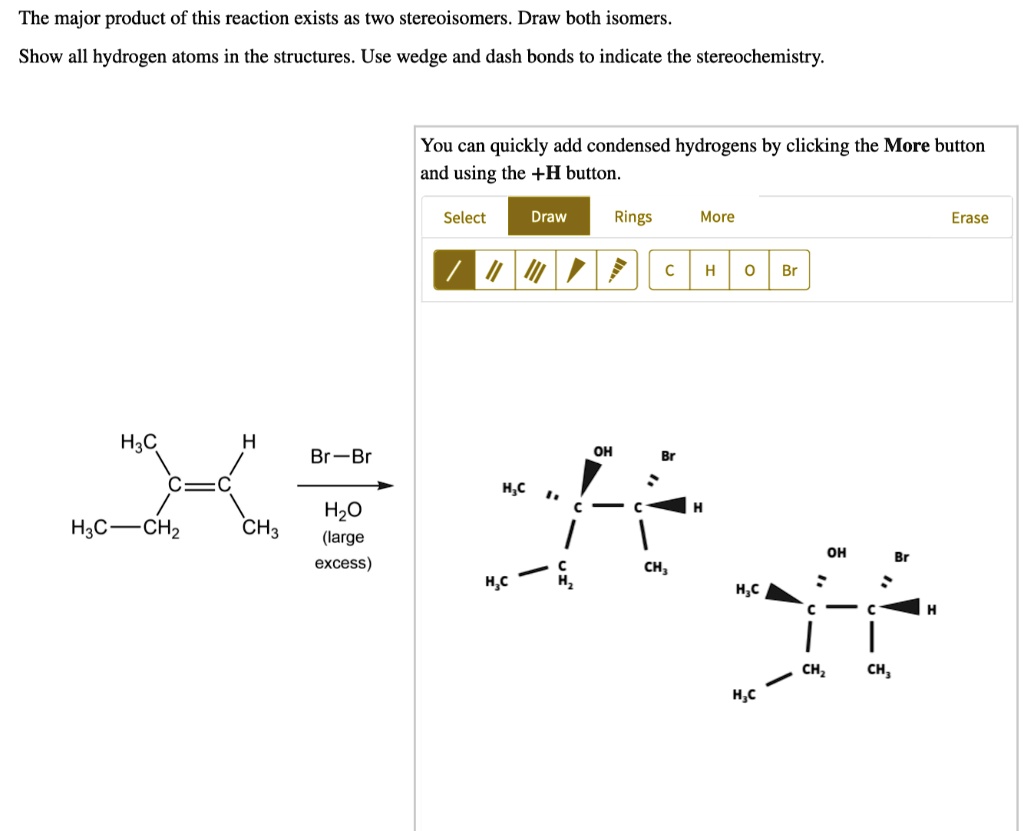
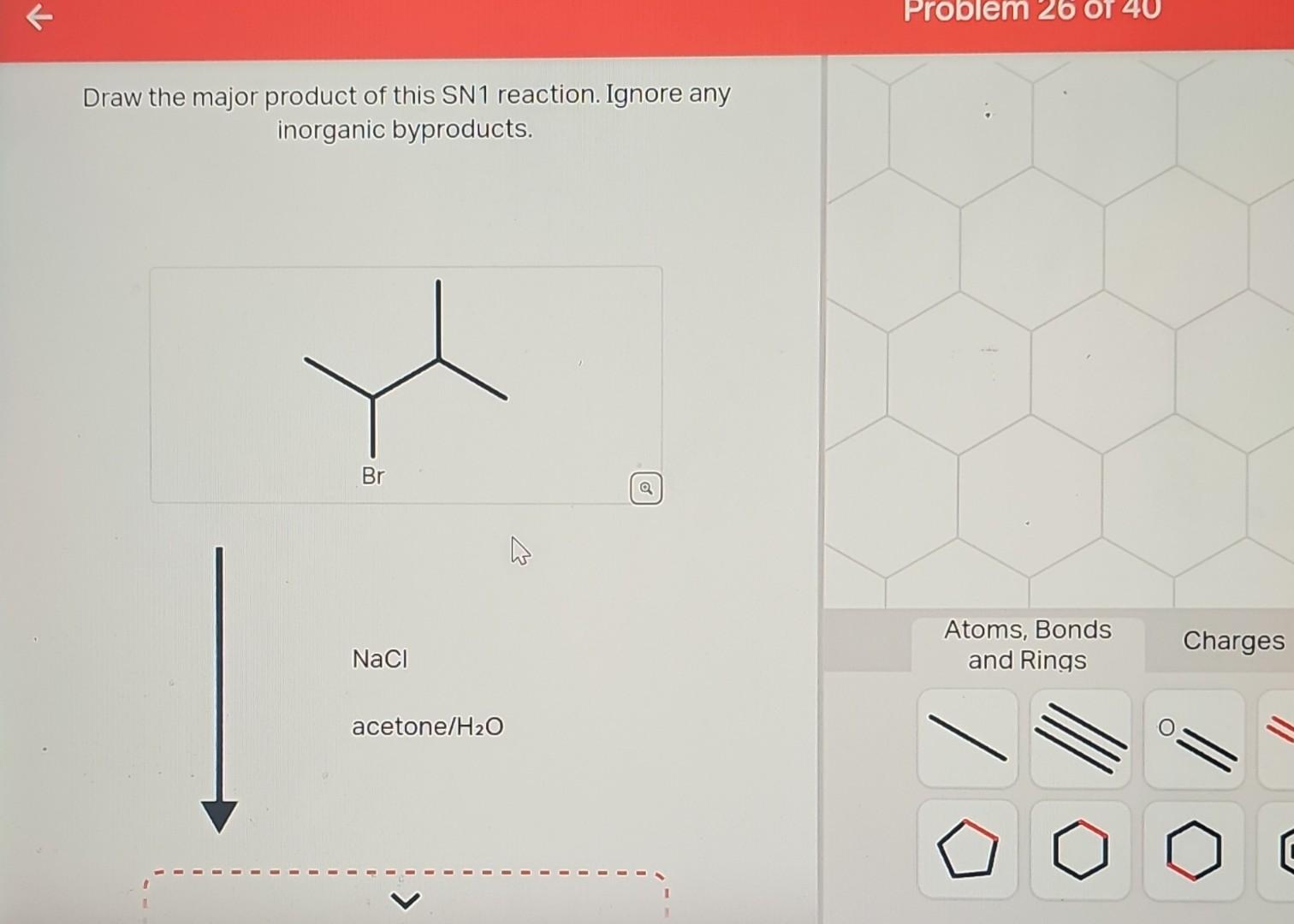
- Reactant: An alkene, specifically 2-methyl-2-butene (a four-carbon chain with a methyl group on the second carbon and a double bond between the second and third carbons). Its structure can be represented as (CH3)2C=CHCH3.
- Reagent: Hydrogen bromide (HBr).
- Solvent: An inert solvent like dichloromethane (CH2Cl2) which does not participate in the reaction.
Understanding the Reaction Mechanism
Once the reactants and reagents are identified, the next step is to understand the reaction mechanism. The mechanism describes the step-by-step process by which the reactants are converted into products. Knowing the mechanism allows us to predict which product is most likely to form.
In this case, we have an alkene reacting with hydrogen bromide (HBr). This reaction is an example of an electrophilic addition. HBr is a strong acid and will donate a proton (H+) to the alkene, which acts as a nucleophile (electron-rich species) due to its double bond. The double bond breaks, and the proton adds to one of the carbons that were part of the double bond. This creates a carbocation intermediate.
Step 1: Protonation of the Alkene
The hydrogen atom from HBr adds to one of the carbons of the double bond in 2-methyl-2-butene. There are two possible carbons for the proton to add to, leading to two different carbocation intermediates. The key here is to consider the stability of the carbocations.
Adding the proton to the carbon-2 (the one with two methyl groups attached) results in a tertiary carbocation. This means the carbon bearing the positive charge is bonded to three other carbon atoms. Adding the proton to carbon-3 (the one with one methyl group and one hydrogen attached) results in a tertiary carbocation as well. In this specific example, both possible carbocations happen to be tertiary, so stability is not the deciding factor at this stage.
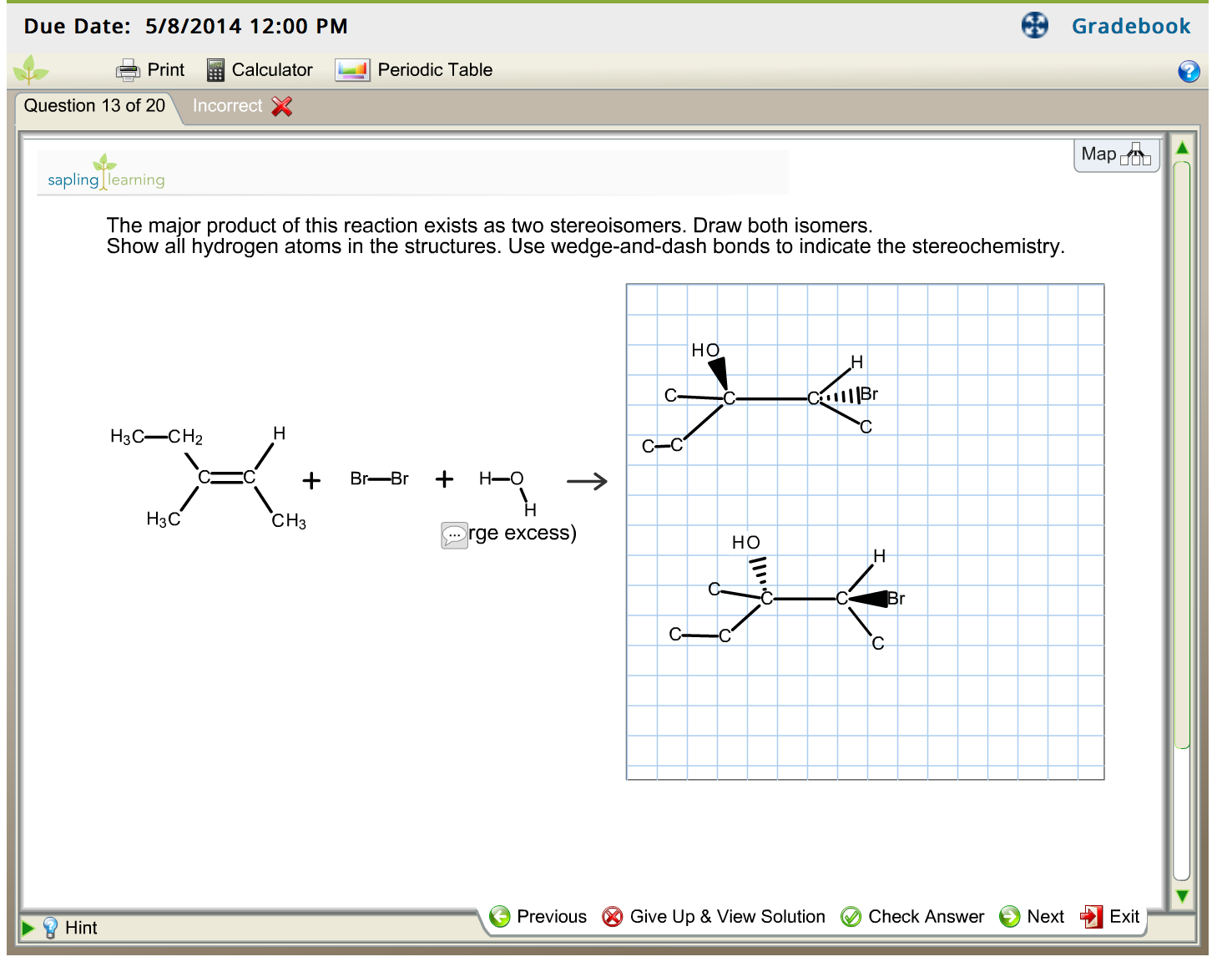
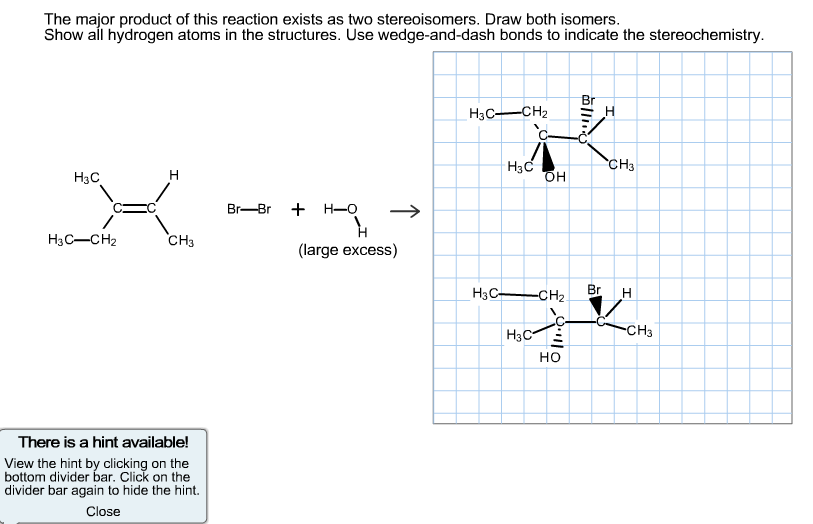
Step 2: Nucleophilic Attack by Bromide Ion
After the formation of the carbocation, the bromide ion (Br-), which is a good nucleophile, attacks the positively charged carbon. This completes the addition reaction, forming an alkyl halide.
Since both carbocations are tertiary, the rate of the nucleophilic attack is the same, and the 2 products will be produced equally, creating a racemic mixture
Predicting the Major Product
Although both carbocations are possible, the relative amounts of each formed are not necessarily equal. Factors like Markovnikov's rule often guide the prediction of the major product. This rule states that in the addition of a protic acid (like HBr) to an alkene, the hydrogen atom adds to the carbon atom that has the greater number of hydrogen atoms already attached. However, Markovnikov's rule is a simplified guideline. The real driving force is the stability of the carbocation intermediate.
In our example, the carbocation intermediate’s stability is the same whether carbon 2 or 3 are protonated. Therefore, the H adds on the carbon that gives the more stable carbocation. If both carbocations are the same stability, then the products will be produced at the same rate.
Considering Regioselectivity
Regioselectivity refers to the preference for a reaction to occur at one region of a molecule over another. In this HBr addition to 2-methyl-2-butene, the regioselectivity is determined by the stability of the carbocation intermediate.
The Importance of Steric Hindrance
Steric hindrance can also play a role in determining the major product. Bulky groups around a reaction center can hinder the approach of reagents, leading to a preference for products where the reagent attacks from the less hindered side. However, in this particular case, steric hindrance is not a significant factor in determining the major product.
Drawing the Major Product
Based on the reaction mechanism and the factors discussed above, we can now draw the structure of the major product.
Since both carbocations are equally stable, then both products should exist as a racemic mixture.
Why This Matters
Predicting the major product of an organic reaction is crucial for several reasons:
- Synthesis Planning: In chemical synthesis, chemists need to know which products will form from a given reaction. Predicting the major product allows them to design efficient synthetic routes to obtain desired compounds.
- Understanding Chemical Behavior: Predicting reaction outcomes enhances our understanding of how molecules interact and transform, leading to better insights into chemical behavior.
- Drug Discovery: In the pharmaceutical industry, accurately predicting reaction products is essential for synthesizing and modifying drug molecules.
- Materials Science: The development of new materials relies on controlled chemical reactions. Knowing the major product ensures the desired material properties are achieved.
- Environmental Chemistry: Predicting the products of chemical reactions is important for understanding the fate of pollutants in the environment.
In conclusion, understanding the reactants, reaction mechanism, and factors influencing product stability is essential for accurately predicting the major product of an organic reaction. Mastering this skill is fundamental for success in organic chemistry and related fields.