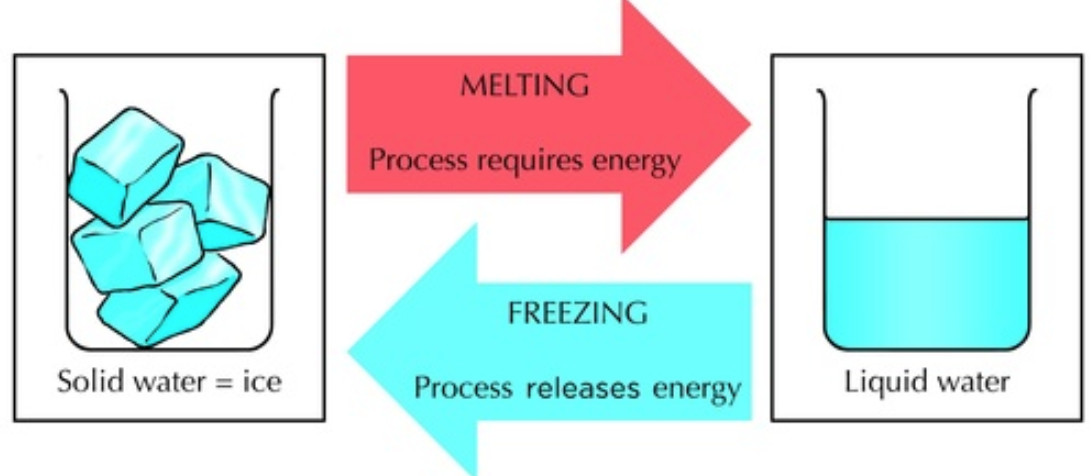
Okay, let's talk about something super fascinating, yet totally everyday: melting ice cubes. You grab one from the freezer, plop it in your drink, and watch it slowly disappear. But have you ever stopped to think about what's actually happening? Specifically, is it a physical change, or something more… dramatic?
Don’t worry, we’re not about to dive into some scary chemistry textbook. We're keeping this chill and exploring the cool science behind this common occurrence. Think of this as a friendly chat over a glass of… water (with an ice cube, naturally!).
What's the Big Deal About Changes Anyway?
So, why even bother classifying changes? Well, understanding the difference between a physical change and a chemical change helps us understand how the world works. It’s like learning the difference between rearranging furniture in your room (physical) and burning your old furniture in a bonfire (chemical – please don't do that!).
Changes are happening all the time. Leaves changing color in autumn, a cake baking in the oven, a puddle evaporating on a hot day… all changes! But they don’t all happen in the same way. Some just alter the appearance or form of a substance, while others create entirely new substances.
Physical Change: The "Superficial" Makeover
A physical change is basically a transformation that doesn't mess with the fundamental identity of a substance. Think of it like getting a haircut. You still have the same hair, just a different style. No new molecules are being created, and no old ones are being destroyed. It's just a rearrangement.
Here are some key characteristics of physical changes:
- The chemical composition stays the same. This is super important!
- The change is often reversible. Think about freezing water back into ice.
- Changes of state are usually physical changes (solid to liquid, liquid to gas, etc.).
- Examples: Crushing a can, dissolving sugar in water, boiling water, tearing paper.
Chemical Change: The Radical Transformation
A chemical change, on the other hand, is a whole different ballgame. This is where things get really interesting! In a chemical change, a substance is transformed into a new substance with different properties. It's like mixing baking soda and vinegar – you get a bubbly reaction and a completely different set of chemicals.
Here are some telltale signs of a chemical change:
- A new substance is formed.
- There's a change in chemical composition.
- The change is often irreversible (or very difficult to reverse).
- You might see bubbles, a color change, or a change in temperature.
- Examples: Burning wood, rusting iron, cooking an egg.
Back to the Ice Cube: A Solid Case for Physical Change
Okay, with that groundwork laid, let's get back to our frosty friend. When an ice cube melts, what's actually happening at the molecular level?
Think of water molecules as tiny, energetic dancers. In solid ice, they're all tightly packed together, barely moving, doing a slow, coordinated waltz. They're locked in place by strong hydrogen bonds, forming a crystal lattice structure. It's organized and rigid.
When you add heat (by taking the ice cube out of the freezer, for example), you're essentially giving these dancers more energy. They start wiggling and jiggling more vigorously. As they gain energy, they break free from those rigid hydrogen bonds.
The crystal lattice structure collapses, and the water molecules are now free to move around more. They're still the same water molecules (H2O), but they're no longer locked in place. They're now doing more of a free-for-all salsa, bumping into each other and flowing around.
So, what does this mean? The water molecules are still water molecules! They haven't transformed into something else. The chemical formula is still H2O. All that’s changed is their arrangement and the state of matter they're in – from solid to liquid.
Therefore, melting an ice cube is a physical change!
Why It's Cool (Pun Intended!)
So, why is this cool? Well, understanding that melting is a physical change helps us understand the basic properties of matter and how energy affects it. It's a fundamental concept in chemistry and physics.
Plus, it illustrates how seemingly simple things can have interesting explanations at the molecular level. You're not just watching ice disappear; you're witnessing the dance of water molecules as they transition from a rigid structure to a fluid state. Isn't that neat?
Think of it this way:
- Physical Change is like: Changing the shape of Play-Doh. You still have Play-Doh, just in a different form.
- Chemical Change is like: Baking a cake. You mix ingredients together, and they react to form something completely new.
- Melting Ice is like: Unpacking a box of LEGO bricks that were all stuck together. The bricks are still the same LEGOs, just no longer connected in a rigid shape.
More Examples of Physical Changes to Ponder:
- Boiling water: Water turns to steam, but it's still water (H2O).
- Crushing a rock: The rock is broken into smaller pieces, but it's still the same rock.
- Dissolving salt in water: The salt disappears visually, but it's still present in the water as individual ions. If you boil off the water, the salt will reappear.
- Bending a paperclip: The shape changes, but the metal is still the same.
Wrapping Up: Embrace the Ordinary (and Extraordinary)
So, the next time you watch an ice cube melt, take a moment to appreciate the simple elegance of this physical change. It's a reminder that even the most ordinary occurrences can be quite extraordinary when you look at them through the lens of science.
Keep asking questions, keep exploring, and keep being curious about the world around you. There's always something new to discover, even in a melting ice cube!
Now, if you'll excuse me, I think I need to refresh my drink. Anyone else want an ice cube?