Understanding the nature of substances and their combinations is fundamental to chemistry and everyday life. One common example is the combination of salt and water. Determining whether salt and water form a mixture or a solution necessitates understanding the definitions of these terms and how they apply to this specific combination.
Defining Key Terms
To begin, we must establish clear definitions for the terms "mixture" and "solution."
Mixture
A mixture is a combination of two or more substances that are physically combined but not chemically bonded. This means each substance retains its individual properties and chemical identity. Mixtures can be separated by physical means, such as filtration, evaporation, or magnetism. There are two main types of mixtures:
- Heterogeneous mixtures: These mixtures have visibly different components. You can easily see the individual substances that make up the mixture.
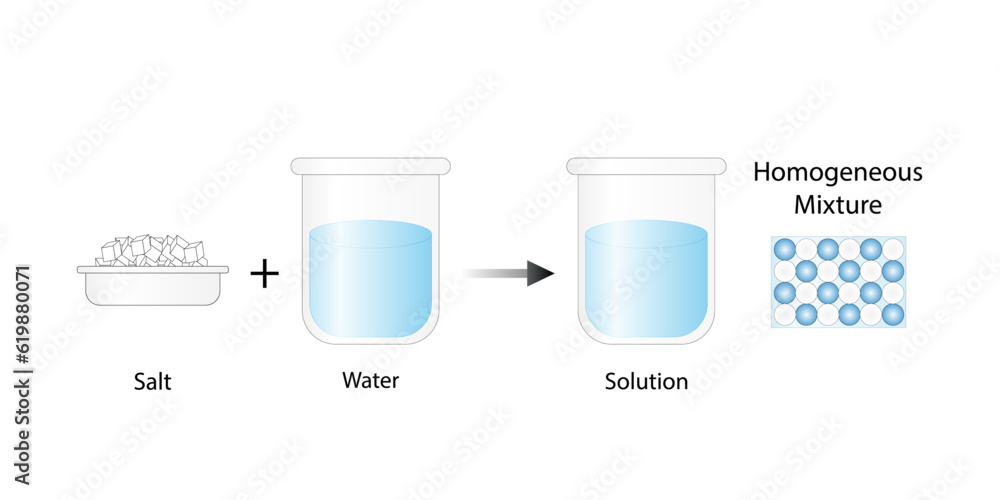
- Homogeneous mixtures: These mixtures have a uniform composition throughout. You cannot distinguish the individual components with the naked eye.
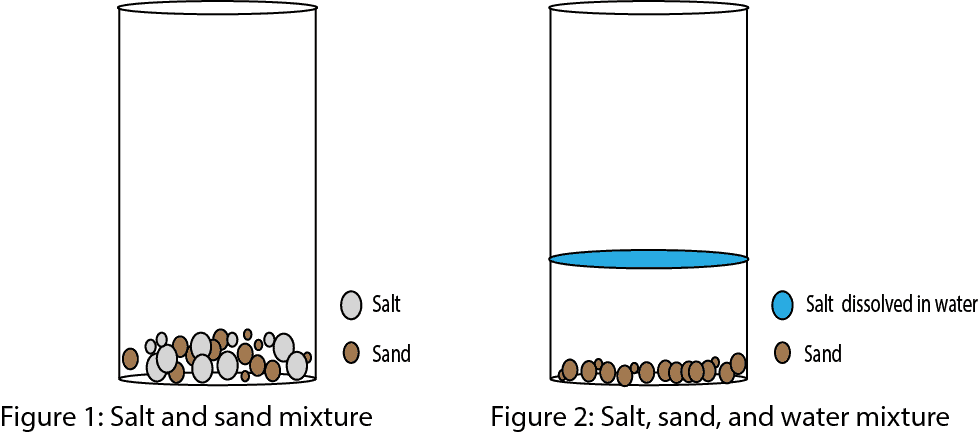
Examples of heterogeneous mixtures include sand and water, oil and water, or a salad. The different ingredients are clearly visible and easily separated. Examples of homogeneous mixtures include air (a mixture of nitrogen, oxygen, and other gases) and metal alloys like bronze (a mixture of copper and tin).
Solution
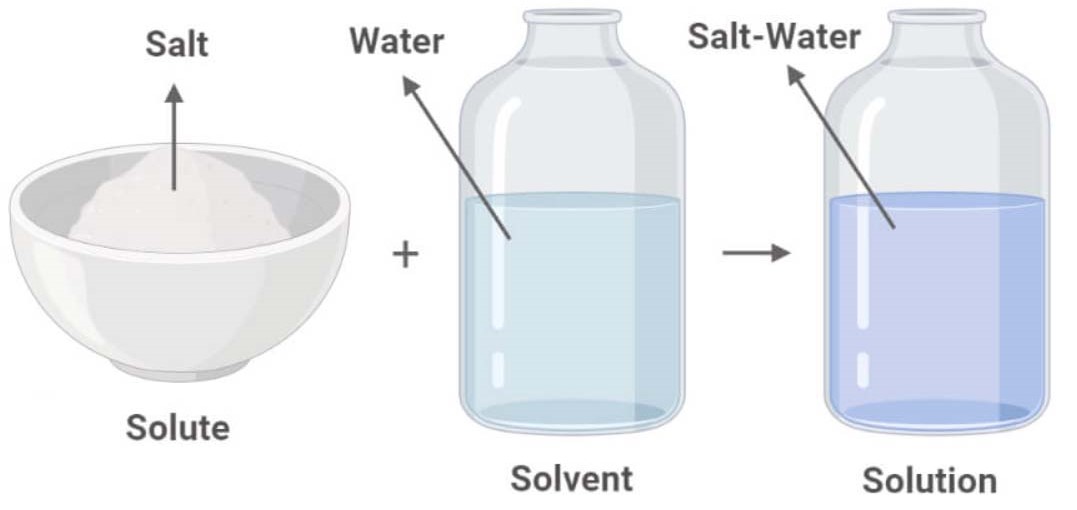
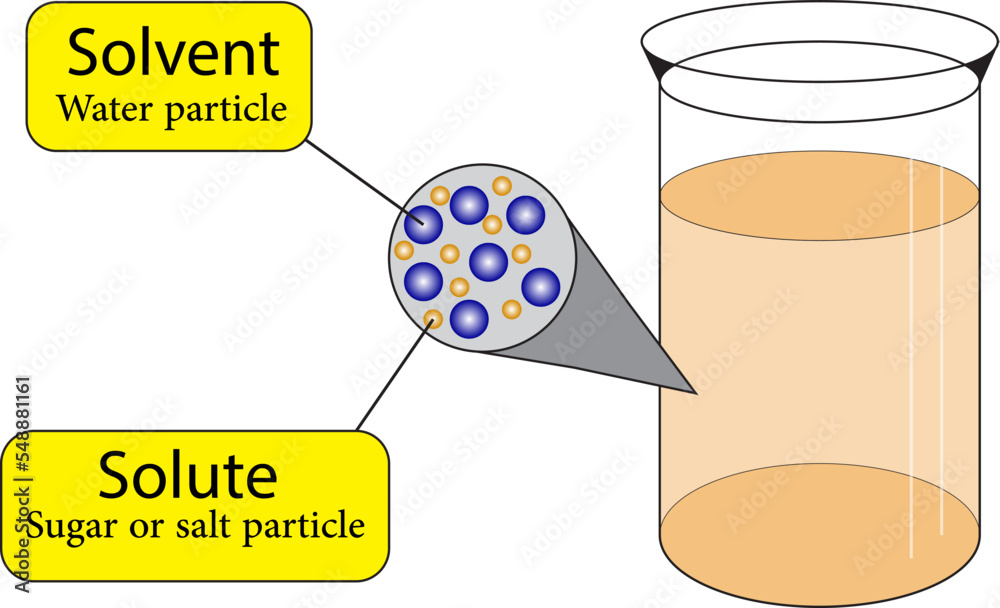
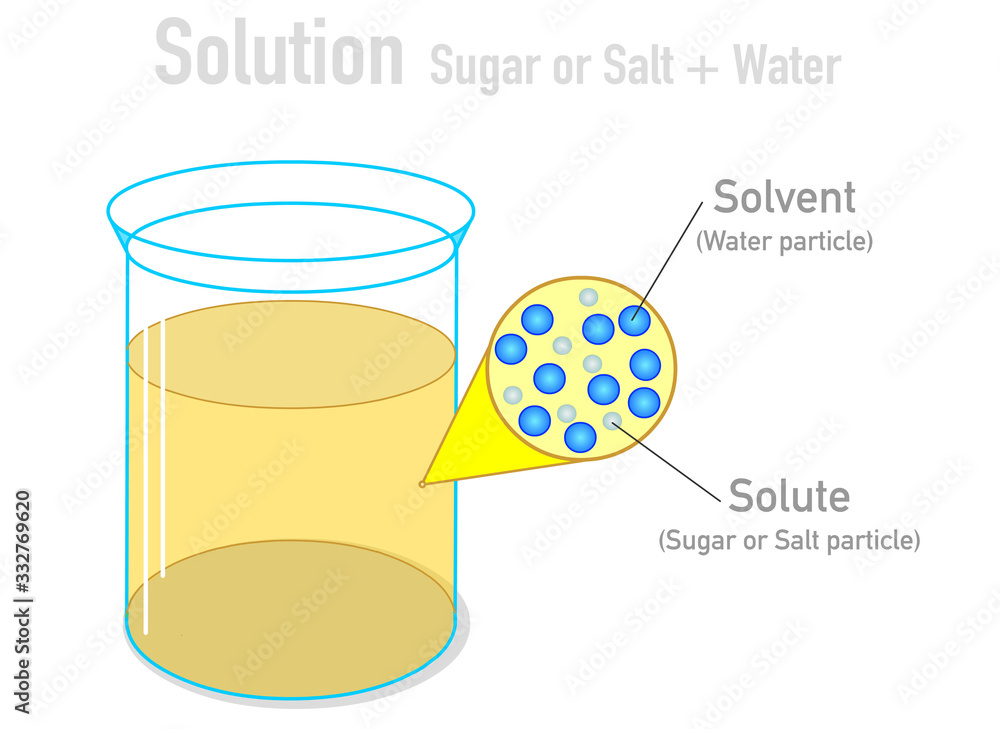
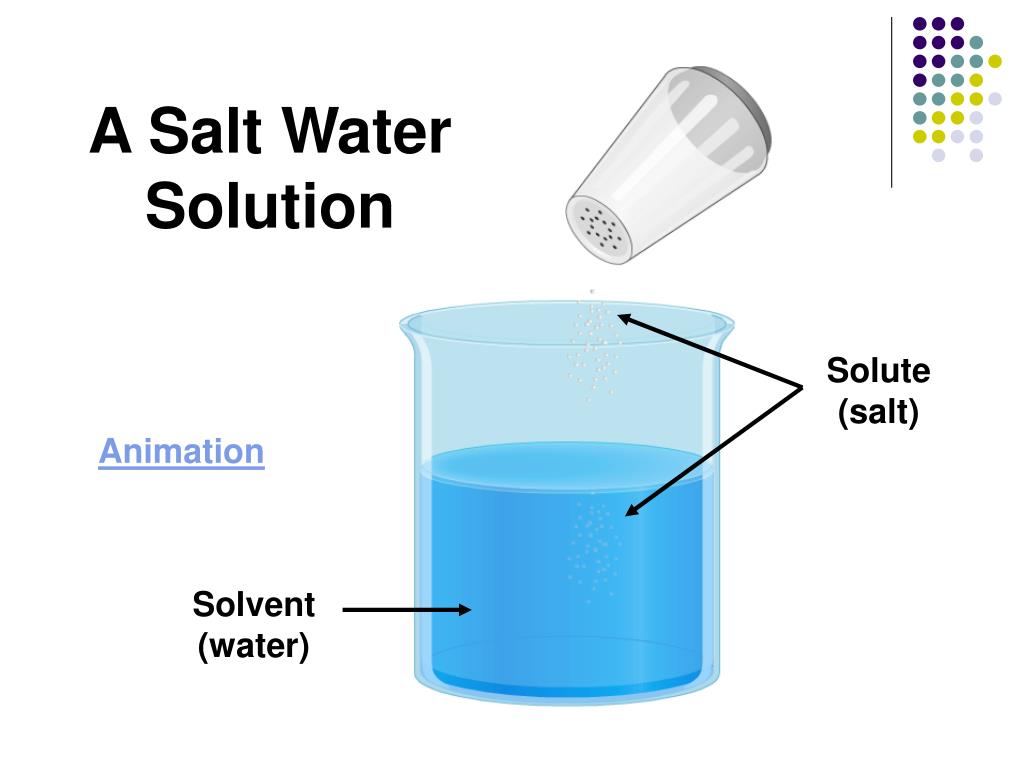
A solution is a specific type of homogeneous mixture where one substance (the solute) is dissolved evenly into another substance (the solvent). The solute is the substance that dissolves, and the solvent is the substance that does the dissolving. In a solution, the solute particles are dispersed uniformly among the solvent particles at a molecular level. Because of this uniform distribution, a solution is always a homogeneous mixture.
Key characteristics of a solution include:
- Homogeneity: The composition is uniform throughout.
- Particle size: The solute particles are very small (ions or molecules) and cannot be seen with the naked eye.
- Stability: The solute does not settle out of the solution over time.
- Transparency: Solutions are typically transparent, meaning light can pass through them without being scattered (although they may be colored).
Common examples of solutions include saltwater (salt dissolved in water), sugar dissolved in water, and carbon dioxide dissolved in water (carbonated beverages).
Salt and Water: A Closer Look
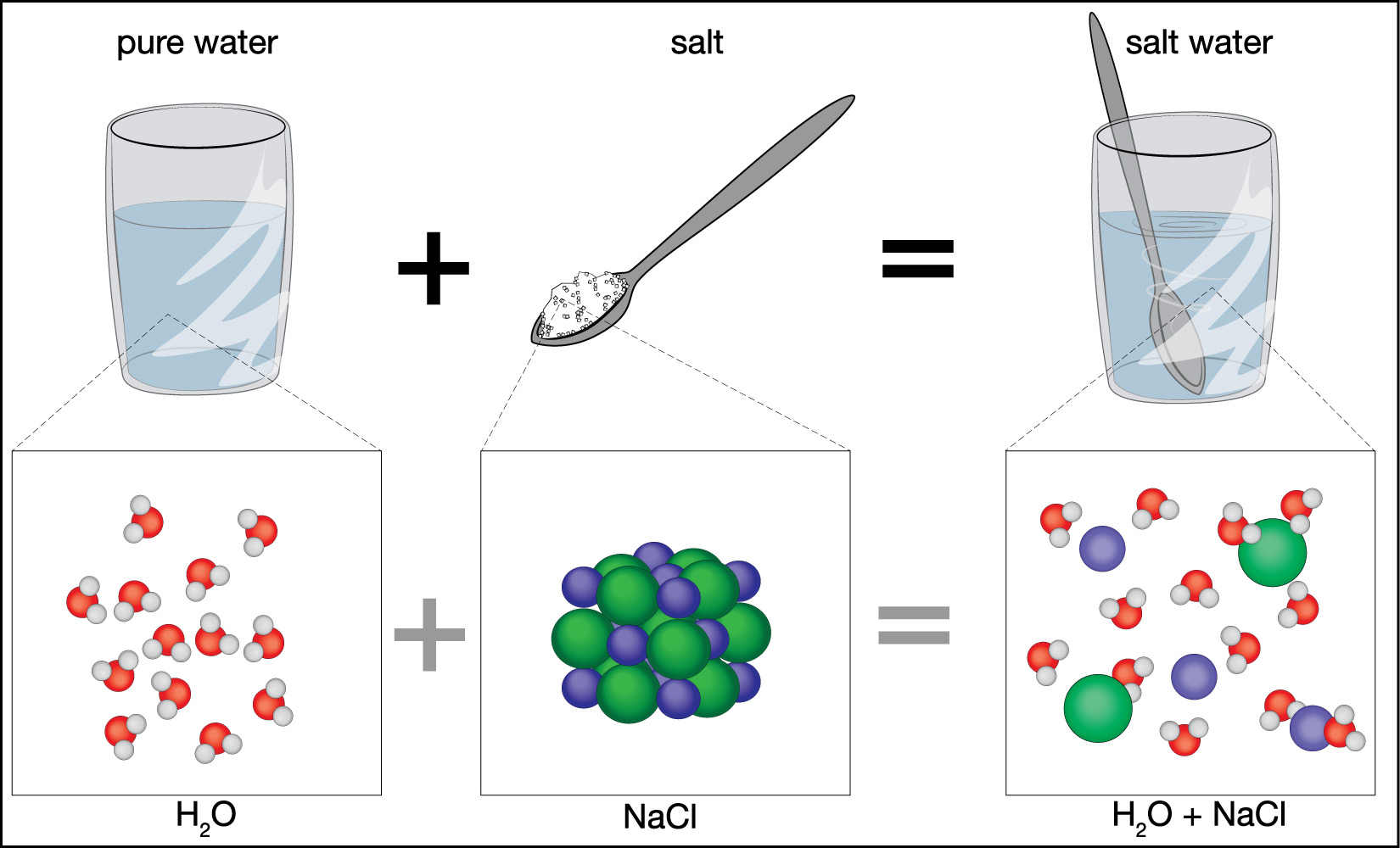
When salt (sodium chloride, NaCl) is added to water (H2O) and stirred, the salt crystals disappear, and the resulting liquid appears clear. At a molecular level, what happens is this:
- Dissociation: Salt is an ionic compound held together by electrostatic forces between positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). When salt is added to water, the polar water molecules surround the ions.
- Solvation: The slightly negative oxygen atoms in water molecules are attracted to the positive sodium ions, and the slightly positive hydrogen atoms in water molecules are attracted to the negative chloride ions. This attraction is stronger than the ionic bonds holding the salt crystal together.
- Dispersion: The water molecules effectively pull apart the sodium and chloride ions, surrounding each ion with a shell of water molecules. This process is called solvation (or hydration when the solvent is water). These hydrated ions are then dispersed evenly throughout the water.
Because the salt ions are dispersed uniformly throughout the water at a molecular level and the mixture exhibits a uniform composition throughout, the resulting mixture of salt and water is a solution. You cannot see the individual salt particles, and they do not settle out over time. The mixture is transparent (unless the salt is impure).
Why Salt and Water is Not Simply a Mixture
While it is true that salt and water are physically combined, classifying them solely as a "mixture" is incomplete and somewhat misleading. The key distinction lies in the homogeneity and the molecular-level interaction between the salt and water.
Consider these points:
- Visibility: In a typical heterogeneous mixture like sand and water, you can easily see the sand particles. In saltwater, you cannot see the salt particles. They are dispersed at the molecular level.
- Settling: Sand particles will settle out of water over time if left undisturbed. Salt ions, however, remain dispersed in water indefinitely under normal conditions.
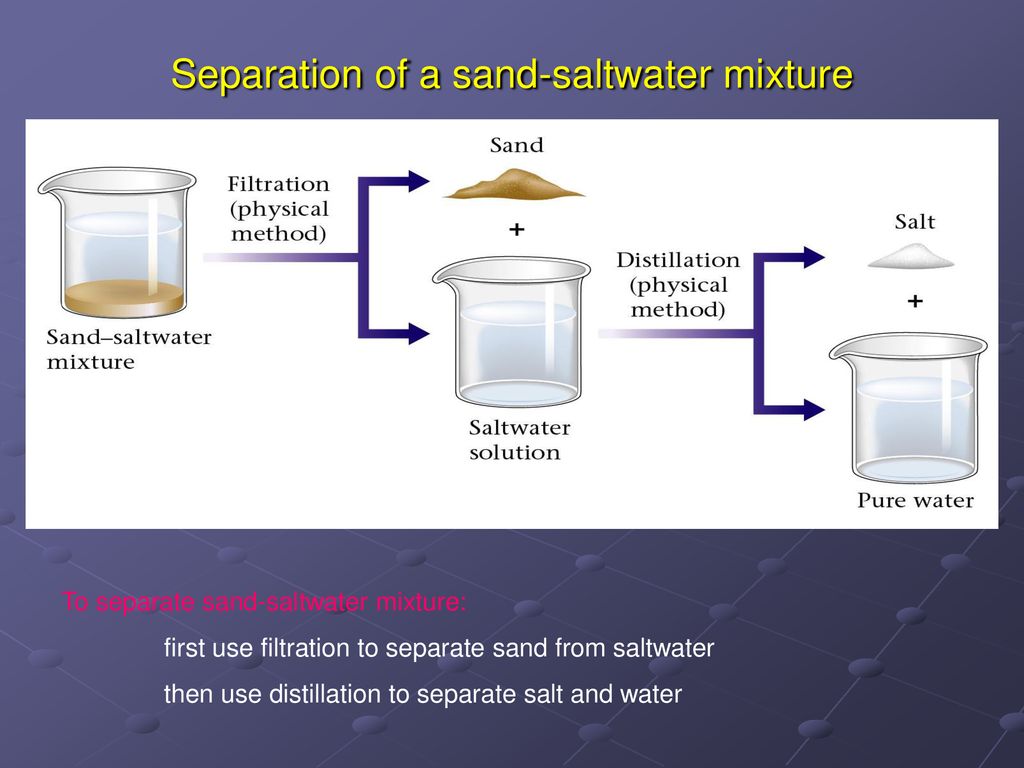
- Separation: While both can be separated by physical means, the ease and method differ. Separating sand from water is as simple as decanting or filtration. Separating salt from water typically involves evaporation, which relies on the different boiling points of the two substances.
Therefore, while technically a solution *is* a type of mixture (specifically a homogeneous mixture), the term "solution" more accurately describes the nature of the salt and water combination due to the uniform dispersion and molecular-level interaction.
Factors Affecting Solubility
The ability of salt to dissolve in water is called its solubility. Solubility is influenced by several factors:
Temperature
Generally, the solubility of solids in liquids increases with temperature. This means that more salt can be dissolved in hot water than in cold water. This is because higher temperatures provide more kinetic energy to the water molecules, allowing them to more effectively break the ionic bonds in the salt crystal and disperse the ions.
Nature of Solute and Solvent
The principle of "like dissolves like" applies. Polar solvents, like water, tend to dissolve polar solutes, like salt. Nonpolar solvents, like oil, tend to dissolve nonpolar solutes, like fats. Salt, being an ionic compound with charged ions, is highly polar and therefore dissolves well in water, which is a polar solvent.
Pressure
Pressure has a negligible effect on the solubility of solids and liquids. It primarily affects the solubility of gases in liquids.
Practical Advice and Insights
Understanding the concept of solutions and mixtures, particularly the salt and water example, has several practical applications in everyday life:
- Cooking: Knowing that salt dissolves more readily in hot water can help you prepare food more efficiently. When making pasta, adding salt to the boiling water ensures that the pasta is seasoned evenly.
- Cleaning: Salt solutions can be used as cleaning agents. For example, a saltwater solution can help remove stains from certain fabrics.
- Gardening: Understanding the solubility of fertilizers is crucial for providing plants with the necessary nutrients.
- Health: Oral rehydration solutions (ORS) used to treat dehydration are based on the principle of creating a solution with the right balance of salts and sugars to facilitate electrolyte absorption.
- Winter Road Safety: Spreading salt on icy roads lowers the freezing point of water, helping to melt the ice and improve road safety. The salt dissolves in the thin layer of water on the ice, forming a solution with a lower freezing point than pure water.
In conclusion, while salt and water can be broadly classified as a mixture, it is more accurately described as a solution due to its homogeneous nature and the uniform dispersion of salt ions within the water at a molecular level. This understanding provides a foundation for comprehending various chemical and physical processes that occur around us daily.





+is+a+compound.+Water+(H20)+is+a+compound..jpg)