Okay, picture this: you're a small biotech company, punching way above your weight class, developing some seriously cool, potentially life-changing drugs. You've got the science down, the early trials are promising, but... you’re running on fumes, financially speaking. Sound familiar? It's the classic David vs. Goliath story, and let's be honest, sometimes David needs a little help from a bigger, stronger friend. That, in a nutshell, is often where licensing agreements come into play. We're talking about Itaca Therapeutics today, and a recent licensing agreement they inked that's got the biotech world buzzing (or at least, quietly murmuring into their overpriced lattes).
What Exactly is a Licensing Agreement? (In Plain English)
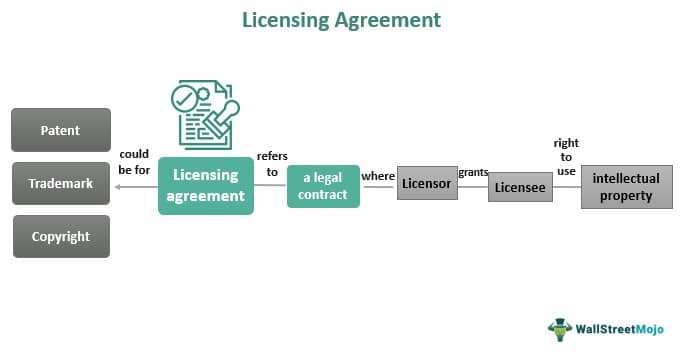
Before we dive into the specifics of Itaca's deal, let's break down what a licensing agreement actually *is*. Think of it like renting out your cool invention to someone who has the resources to really make it shine. You, the inventor (in this case, Itaca), retain ownership of the invention (the drug), but you grant another company (usually a much larger pharmaceutical company, with deep pockets and a sprawling sales force) the right to develop, manufacture, and sell it.
Why do this? Well, for the smaller company, it's a lifeline. They get an influx of cash, expertise, and access to markets they could only dream of before. For the bigger company, it's a way to beef up their pipeline, diversify their portfolio, and potentially score a blockbuster drug without having to invest all the upfront research and development costs themselves. Win-win, right? (Well, potentially win-win. The devil, as always, is in the details.)
Itaca Therapeutics: A Quick Intro
So, who are these Itaca folks anyway? They're a relatively young company focused on developing therapies for rare genetic diseases. And, let's be frank, rare disease research is *tough*. It's expensive, the patient populations are small, and regulatory hurdles can be high. But it’s also incredibly rewarding when you can impact lives. That's where these licensing agreements can be really impactful, by speeding up the drug development process.
We should take a moment to acknowledge how tricky it can be for smaller players to bring these innovative therapies to patients. It requires an incredible amount of grit, determination, and, crucially, funding. Itaca's licensing agreement is a testament to the innovative work they're doing.
The Deal: What We Know (and What We Can Guess)
Alright, let's get down to the nitty-gritty. What exactly did Itaca license, and to whom? Without giving away any inside information (because, you know, NDAs are a thing!), we can look at the publicly available details, which often include the following key elements:
- The Drug/Technology: The specific therapeutic candidate or technology being licensed. Often, it will focus on the specific diseases or conditions the drug aims to treat.
- The Licensee: The pharmaceutical company acquiring the rights. Knowing who they are can give you insight into their strategy and priorities.
- Territory: The geographical area where the licensee has the rights to develop and market the drug (e.g., North America, Europe, Global).
- Financial Terms: This is the juicy part! It typically includes:
- Upfront Payment: A lump sum paid to Itaca upon signing the agreement. This is like "rent" for the asset.
- Milestone Payments: Additional payments triggered by achieving specific milestones, such as successful clinical trials, regulatory approvals, or sales targets. These milestones are crucial! They show the drug is moving through the pipeline.
- Royalties: A percentage of the future sales of the drug paid to Itaca. This is where the long-term payoff lies.
- Development and Commercialization Responsibilities: Who is responsible for conducting further clinical trials, manufacturing the drug, and marketing it? (Hint: usually the licensee.)
The exact financial terms are usually kept confidential, but analysts and industry experts often try to estimate the potential value of the deal based on similar agreements and the market potential of the drug. It's all a big guessing game, but it can be a fun guessing game!
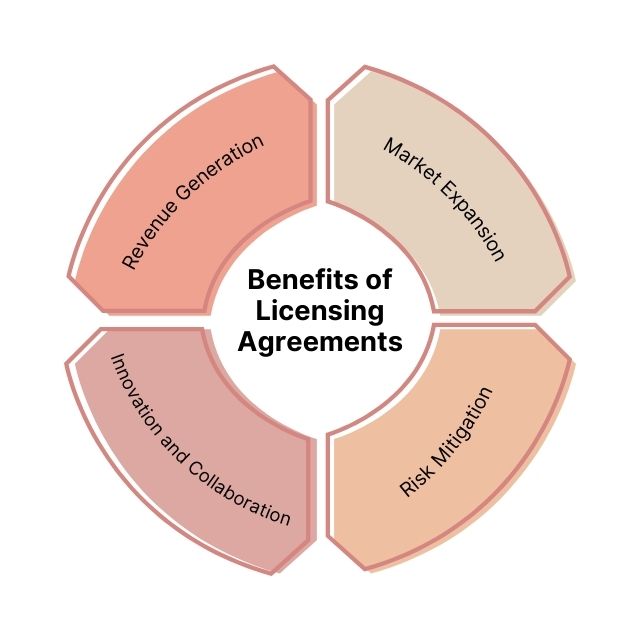
Why This Deal Matters (Beyond the Money)
So, what’s the big deal about this particular agreement? It's not just about the money (although, let's be real, the money *does* matter). It's about the potential to bring a much-needed therapy to patients who are currently underserved. When a bigger company with the resources and expertise of, say, a Pfizer or Novartis gets involved, it significantly increases the odds that the drug will successfully navigate the regulatory process and reach the market. This can provide hope and make an actual difference to patients and their families. What’s more important than that?
Here's a breakdown of why this licensing agreement is worth paying attention to:
- Validation of Itaca's Technology: A licensing agreement with a major pharmaceutical company is a huge vote of confidence in Itaca's science and development capabilities. It says, "Hey, we think you're onto something really special!"
- Accelerated Development: The licensee likely has the resources and expertise to conduct clinical trials more efficiently and navigate the regulatory process faster. This means potentially getting the drug to patients sooner.
- Expanded Reach: The licensee's global reach allows the drug to be made available to patients in more countries, increasing its impact on the lives of those who need it.
- Future Opportunities: A successful licensing agreement can open doors to future partnerships and collaborations for Itaca, further fueling their research and development efforts.
The Risks and Challenges (Let's Not Get Too Carried Away)
Now, let's not paint too rosy a picture. Licensing agreements are not without their risks. There are potential pitfalls to consider:
- Loss of Control: Once the drug is licensed, Itaca has less control over its development and commercialization. They're essentially entrusting their "baby" to someone else.
- Milestone Dependence: A significant portion of the financial benefits may be tied to achieving specific milestones. If the drug fails to meet those milestones (e.g., fails a clinical trial), Itaca may not receive the full value of the agreement.
- Royalty Disputes: Calculating and verifying royalties can be complex and lead to disputes between the licensor and licensee. Nobody wants a legal battle!
- Licensee Priorities: The licensee's priorities may shift over time, and they may decide to deprioritize the licensed drug if they have other more promising candidates in their pipeline. This is brutal, but it happens.
It's important to remember that the pharmaceutical industry is notoriously unpredictable. A drug that looks promising in early clinical trials can still fail in later stages. And even if a drug is approved, it may not achieve the commercial success that was initially anticipated. But Itaca are taking all the right steps in protecting their intellectual property.
What Does This Mean for the Future of Itaca Therapeutics?
This licensing agreement is a major milestone for Itaca Therapeutics. It provides them with the resources they need to continue their research and development efforts and potentially bring life-changing therapies to patients. It validates their innovative science and positions them for future success.
But this isn't the finish line; it's more like the starting gun for the next race. Itaca will need to carefully manage the relationship with their licensee and ensure that the drug continues to progress through the development pipeline. They'll also need to continue to innovate and develop new therapies to stay ahead of the curve. In other words, it's back to the lab!
Here are some potential future scenarios for Itaca:
- Continued Success: The licensed drug successfully navigates clinical trials, obtains regulatory approval, and becomes a commercial success. This generates significant revenue for Itaca through royalties and positions them as a leader in their field.
- Further Partnerships: The success of the licensing agreement attracts interest from other pharmaceutical companies, leading to further partnerships and collaborations.
- Acquisition: A larger pharmaceutical company may acquire Itaca outright to gain access to their pipeline and technology. This is the "exit strategy" that many biotech companies dream of.
- Challenges and Setbacks: The licensed drug encounters unexpected challenges during development, such as adverse side effects or lack of efficacy. This could delay or even halt the drug's progress.
The Bigger Picture: Licensing and Innovation
Itaca Therapeutics' licensing agreement is a microcosm of a larger trend in the pharmaceutical industry: the increasing reliance on partnerships and collaborations to drive innovation. Big Pharma companies are realizing that they can't do it all themselves. They need to tap into the expertise and innovation of smaller biotech companies to fill their pipelines and stay competitive. Licensing agreements are, therefore, becoming increasingly common and important for advancing medical research.
Ultimately, licensing agreements are about bringing innovative therapies to patients who need them. They're a critical mechanism for translating scientific discoveries into real-world treatments. And while there are risks and challenges involved, the potential rewards – both financial and societal – are enormous. So, the next time you hear about a licensing agreement in the biotech world, remember that it's not just about the money. It's about the potential to save lives and improve the human condition. Pretty cool, right?
So keep an eye on Itaca Therapeutics. Their story is just beginning, and it's one that could have a significant impact on the future of medicine. And that’s a story worth following.




![Free Printable License Agreement Templates [Word, PDF] Software - "itaca Therapeutics" Licensing Agreement](https://www.typecalendar.com/wp-content/uploads/2023/05/License-Agreement-1-1200x675.jpg)