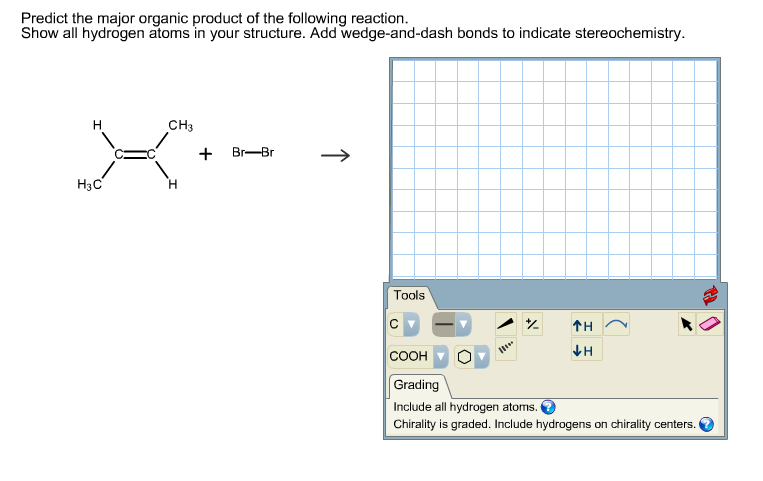
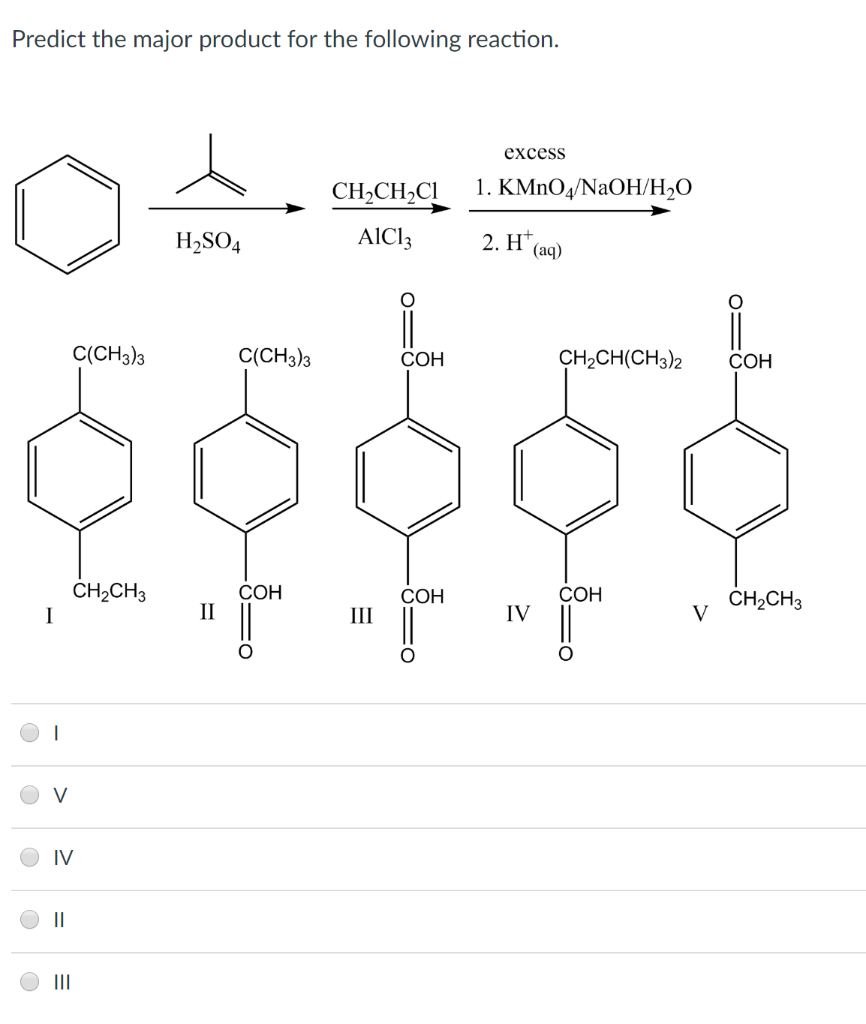
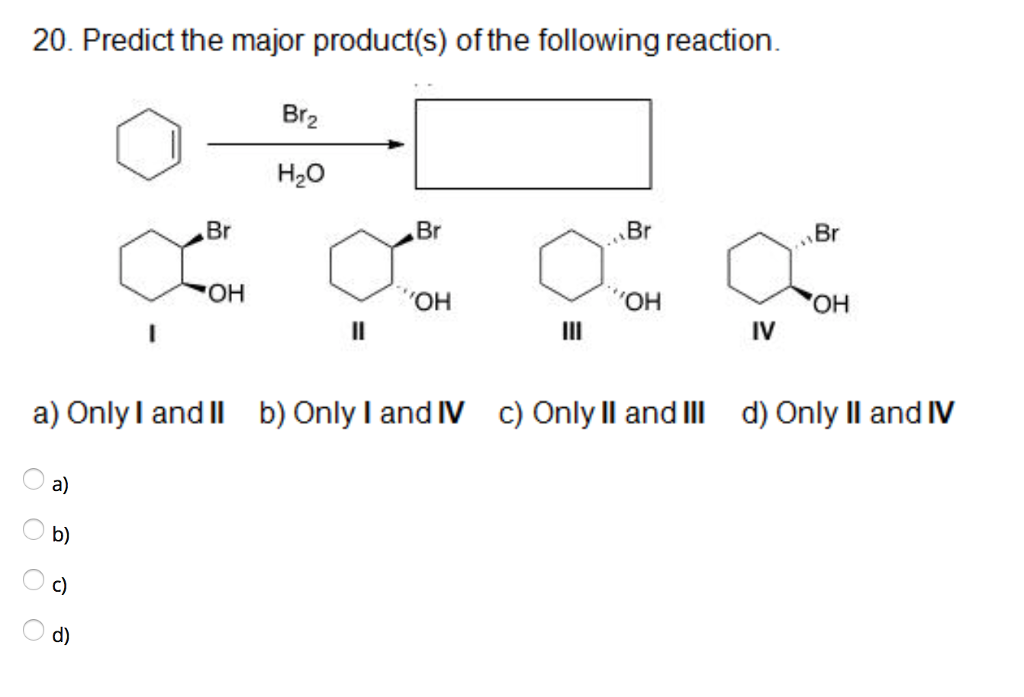
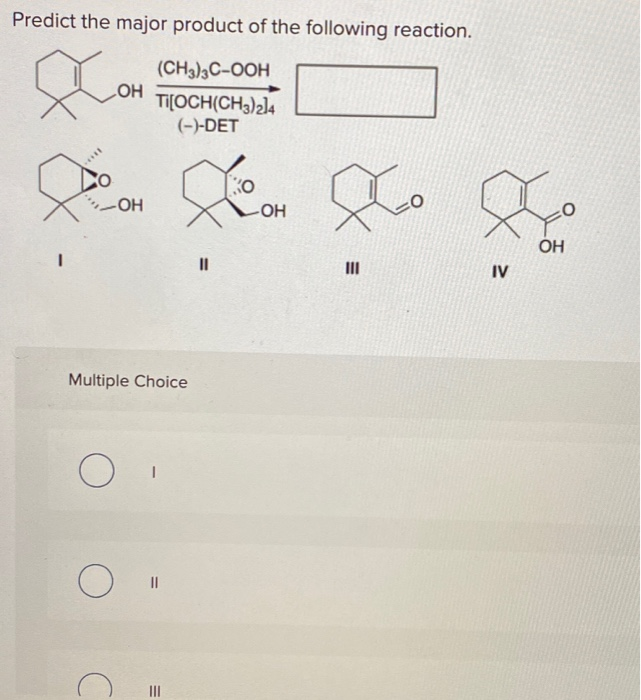
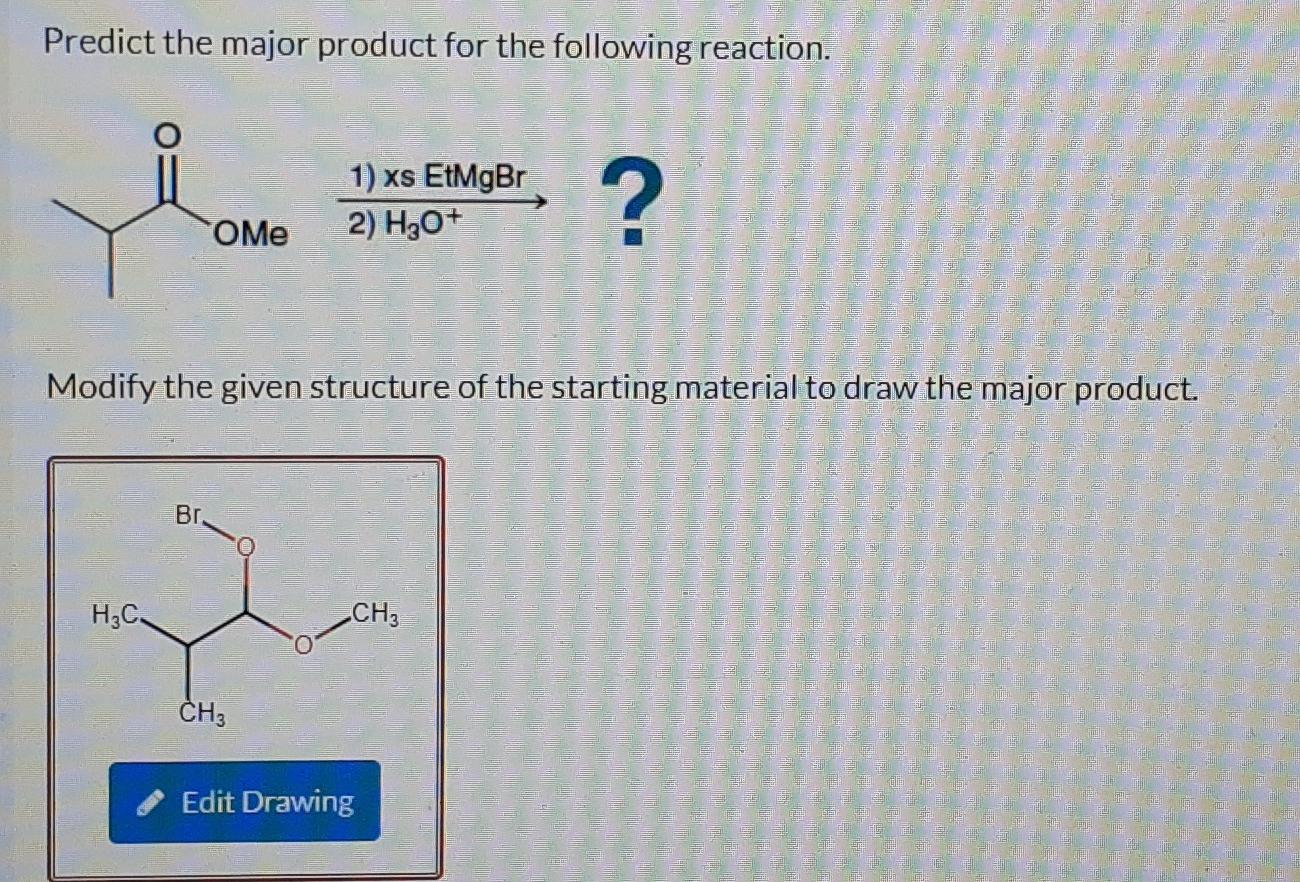
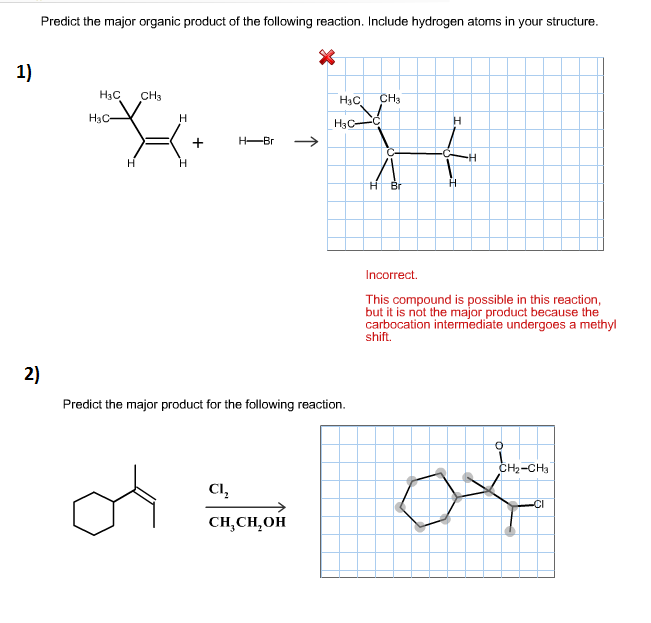
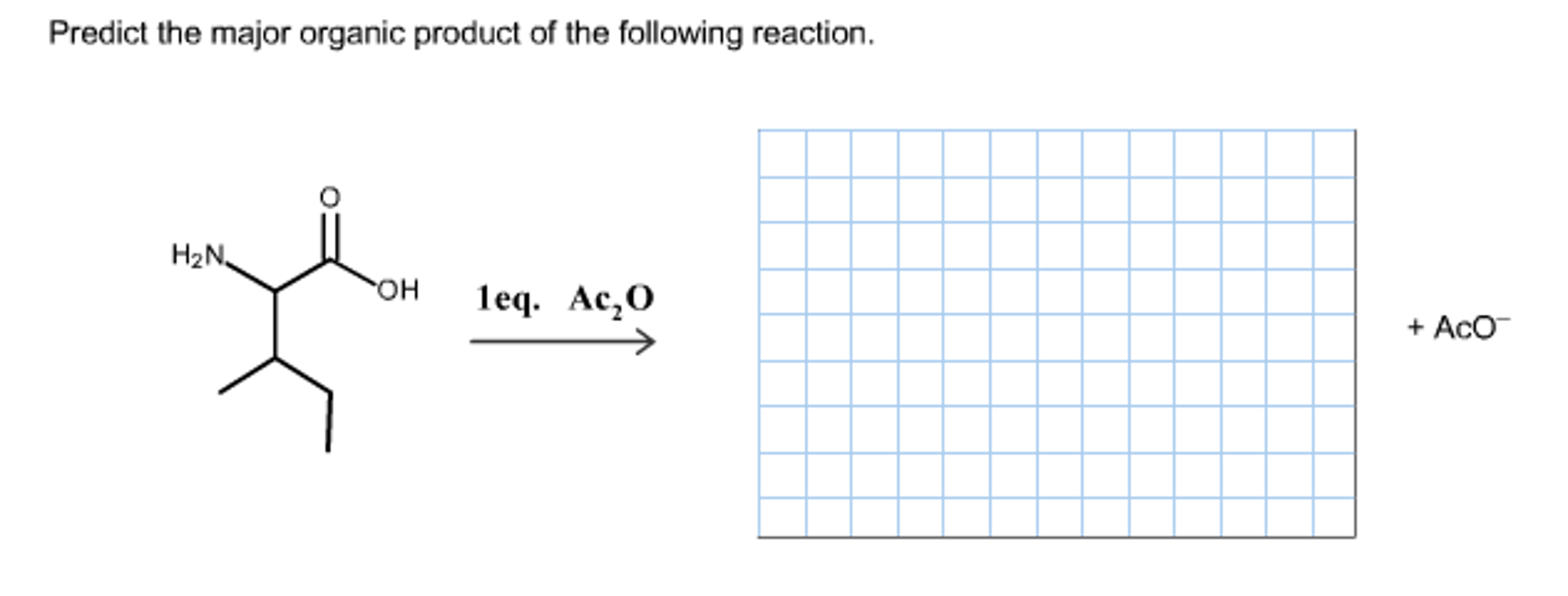
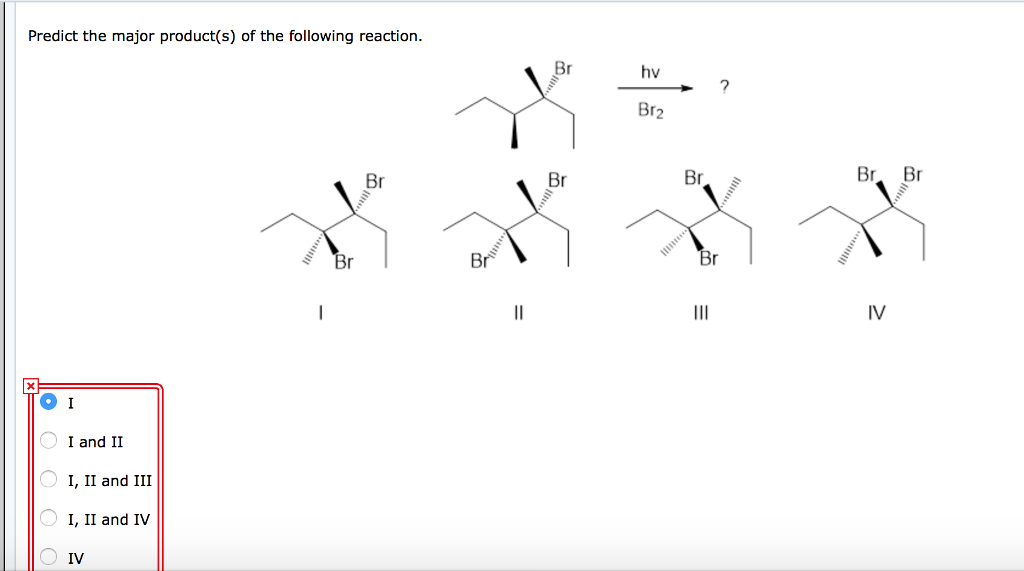
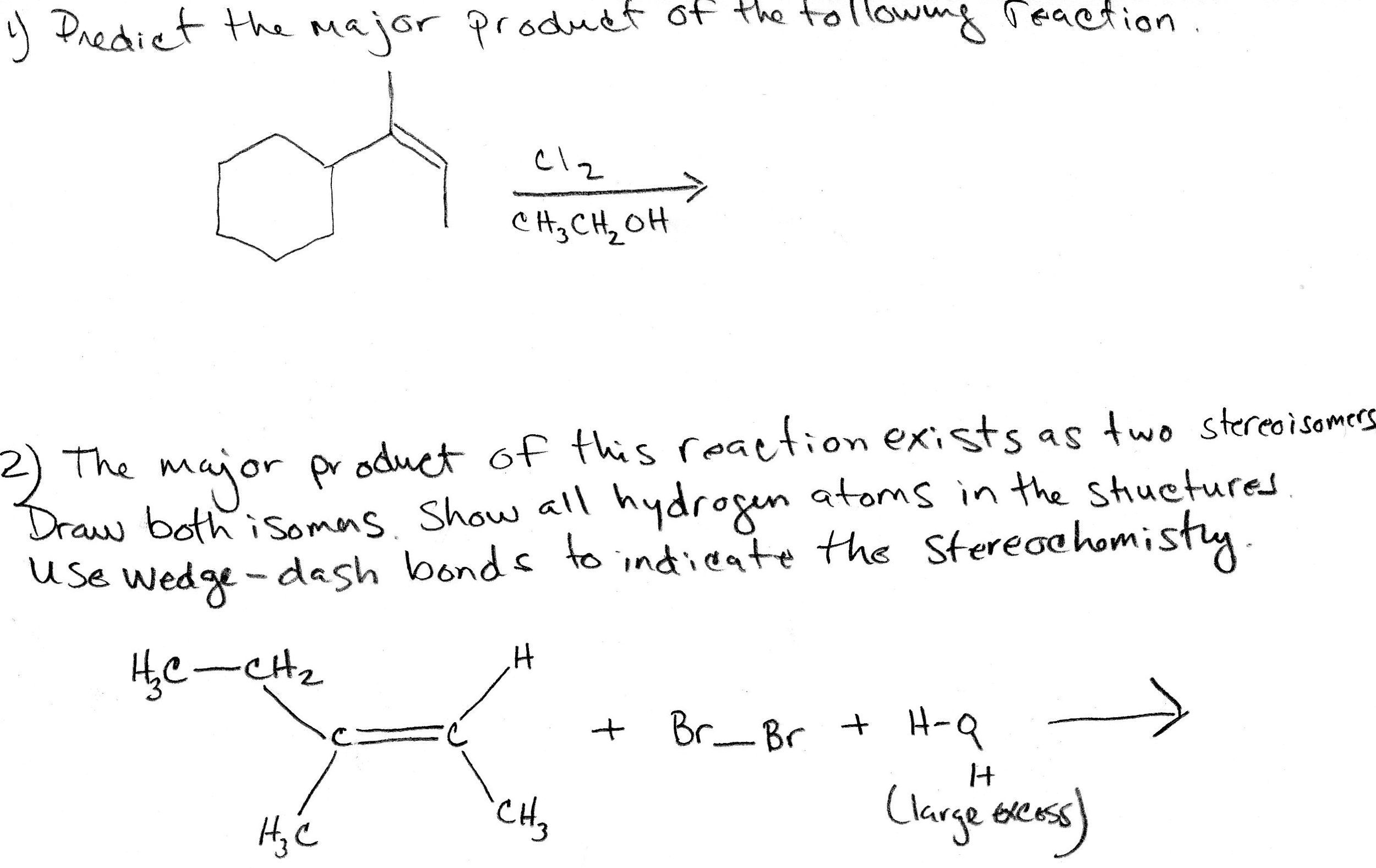
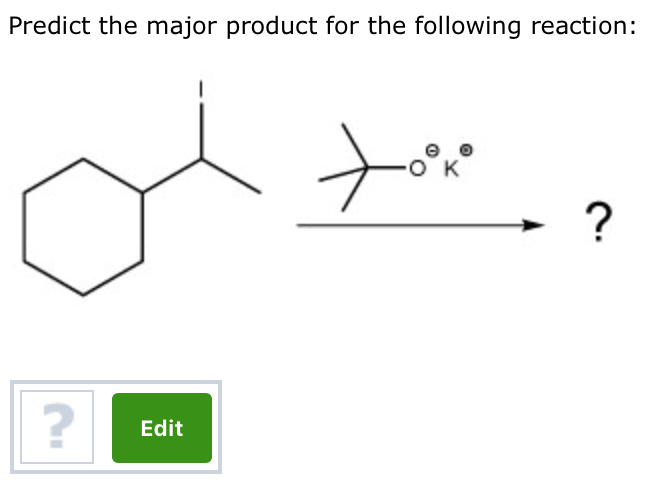
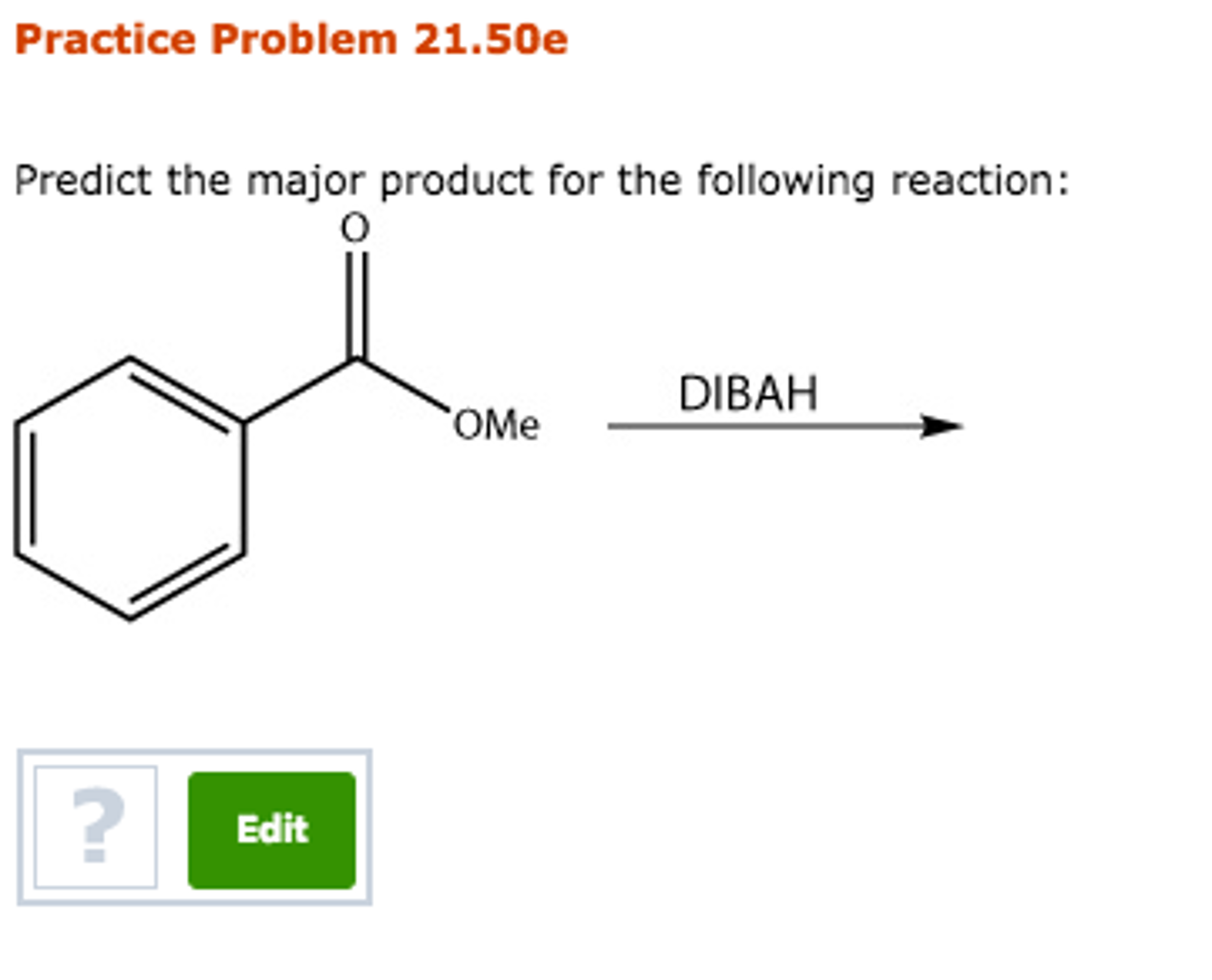
Alright, chemistry enthusiasts! Let's dive into a fascinating little reaction puzzle. We're going to try and predict the major product of a specific chemical dance. Don't worry if the words sound a bit intimidating. We'll break it down, make it fun, and by the end, you'll be saying, "Hey, I kinda get this!"
The Reaction: A Sneak Peek
Now, because I can't *actually* draw out the reaction here, let's imagine something similar. Picture this: We have a molecule, let’s call it Molecule A. Molecule A has a double bond – think of it like two hands holding onto each other really tightly. Then, we introduce something called HBr (hydrobromic acid). HBr is like a pushy dancer at a party, eager to join in on the double-bond action.
So, the big question is: what happens when Molecule A and HBr get together? Where does the Br (bromine) end up attaching? Is there a specific spot it *prefers*? That's what we're figuring out today! It's all about understanding the rules of the chemical dance floor.
Why is This Interesting Anyway?
Okay, so why should you care about predicting reaction products? Well, think of it like this:
- Drug Design: Knowing how molecules react helps scientists design new medicines. If you can predict how a drug will behave in the body, you're one step closer to curing diseases.
- Material Science: Creating new plastics, polymers, and other materials relies heavily on predicting reactions. Want a super-strong, flexible phone case? Chemistry is the answer!
- Understanding Life: Life itself is just a series of incredibly complex chemical reactions. Understanding those reactions is key to understanding biology.
Basically, predicting reaction outcomes is like having a superpower in the molecular world. You can control the dance, orchestrate the connections, and build things that were never possible before. Pretty cool, right?
The Key Player: Markovnikov's Rule
Alright, let's introduce our star player: Markovnikov's Rule. This rule is like the bouncer at the chemical nightclub. It dictates where the bromine (Br) is most likely to attach itself. In simple terms, Markovnikov's Rule says:
"The hydrogen (H) from HBr attaches to the carbon in the double bond that already has more hydrogens attached to it."
Whoa, that sounds a bit complicated, doesn't it? Let's break it down further. Imagine those two carbons in the double bond are like two friends at school. One friend (let's call him Carbon Bob) already has a lot of other friends (hydrogens) hanging around him. The other friend (Carbon Alice) is a bit more of a loner, with fewer friends (hydrogens).
Markovnikov's Rule basically says that the new hydrogen from HBr is more likely to hang out with Carbon Bob (the one who already has more friends). And because the bromine (Br) and hydrogen (H) are a package deal, that means the bromine will end up attaching to Carbon Alice, the loner.
Applying the Rule: Let's Get Specific (Hypothetically!)
Let's pretend Molecule A is propene (CH3-CH=CH2). Now, we're adding HBr. Let’s focus on the two carbons involved in the double bond (CH=CH2).
- Carbon #1 (the one connected to the CH3 group) has one hydrogen directly attached to it.
- Carbon #2 (the end carbon) has two hydrogens directly attached to it.
According to Markovnikov's Rule, the hydrogen from HBr will attach to carbon #2 (because it has more hydrogens already), and the bromine will attach to carbon #1. Therefore, the major product would be 2-bromopropane (CH3-CHBr-CH3).
See? It's not so scary! Identify the double bond, count the hydrogens on each carbon, and let Markovnikov's Rule guide you. It's like following a recipe, but for molecules!
Why Does Markovnikov's Rule Work? The Carbocation Story
Okay, this is where things get a *little* bit more advanced, but stay with me. The reason Markovnikov's Rule works is due to something called a carbocation. A carbocation is basically a carbon atom with a positive charge. It's like a carbon that's missing an electron and is feeling a bit unstable.
When HBr approaches the double bond, the first step is that the double bond grabs a hydrogen (H+) from HBr. This forms a carbocation on one of the two carbons that were part of the double bond. Now, here's the crucial part:
The more stable carbocation is the one that forms!
And which carbocation is more stable? The one that's attached to more carbon groups. Think of it like this: carbon groups are like little electron-donating friends that help stabilize the positive charge on the carbocation. The more friends (carbon groups) it has, the happier and more stable the carbocation is.
So, in our propene example, when the double bond grabs a hydrogen, it's more likely to form a carbocation on the central carbon (carbon #1) because that carbon is attached to two other carbon groups (the CH3 group and another CH group). This is called a secondary carbocation.
If the carbocation formed on the end carbon (carbon #2), it would only be attached to one other carbon group (the central CH group). This is called a primary carbocation.
Secondary carbocations are more stable than primary carbocations. And because the reaction prefers to form the more stable carbocation, it will favor the pathway that leads to the bromine attaching to the central carbon, resulting in 2-bromopropane.
Therefore, while Markovnikov's Rule seems like a simple rule, it's actually based on the underlying principles of carbocation stability!
Exceptions to the Rule (Because Chemistry Loves to Confuse You!)
Just when you thought you had it all figured out, chemistry throws you a curveball! There are exceptions to Markovnikov's Rule, often involving special conditions or other chemical players. For example, sometimes, under specific conditions like using peroxides, the reaction can proceed via an anti-Markovnikov pathway. This means the bromine ends up attaching to the carbon with *more* hydrogens, which is the opposite of what Markovnikov's Rule predicts.
Think of it like this: sometimes the bouncer gets bribed, and the rules of the chemical nightclub get temporarily suspended. However, for most simple reactions with HBr and alkenes, Markovnikov's Rule is a pretty reliable guide.
In Summary: You Can Do This!
Predicting the major product of a reaction like this might seem daunting at first, but with a little bit of understanding and a dash of practice, you can become a pro. Remember:
- Identify the double bond.
- Count the hydrogens on each carbon involved in the double bond.
- Apply Markovnikov's Rule (hydrogen goes to the carbon with more hydrogens already).
- Consider the carbocation stability.
- Be aware that there are exceptions!
Chemistry is like learning a new language. It takes time and effort, but the more you practice, the more fluent you become. So, keep exploring, keep asking questions, and keep challenging yourself. And who knows, maybe you'll be the one designing the next generation of life-saving drugs or super-strong materials!
Happy reacting!