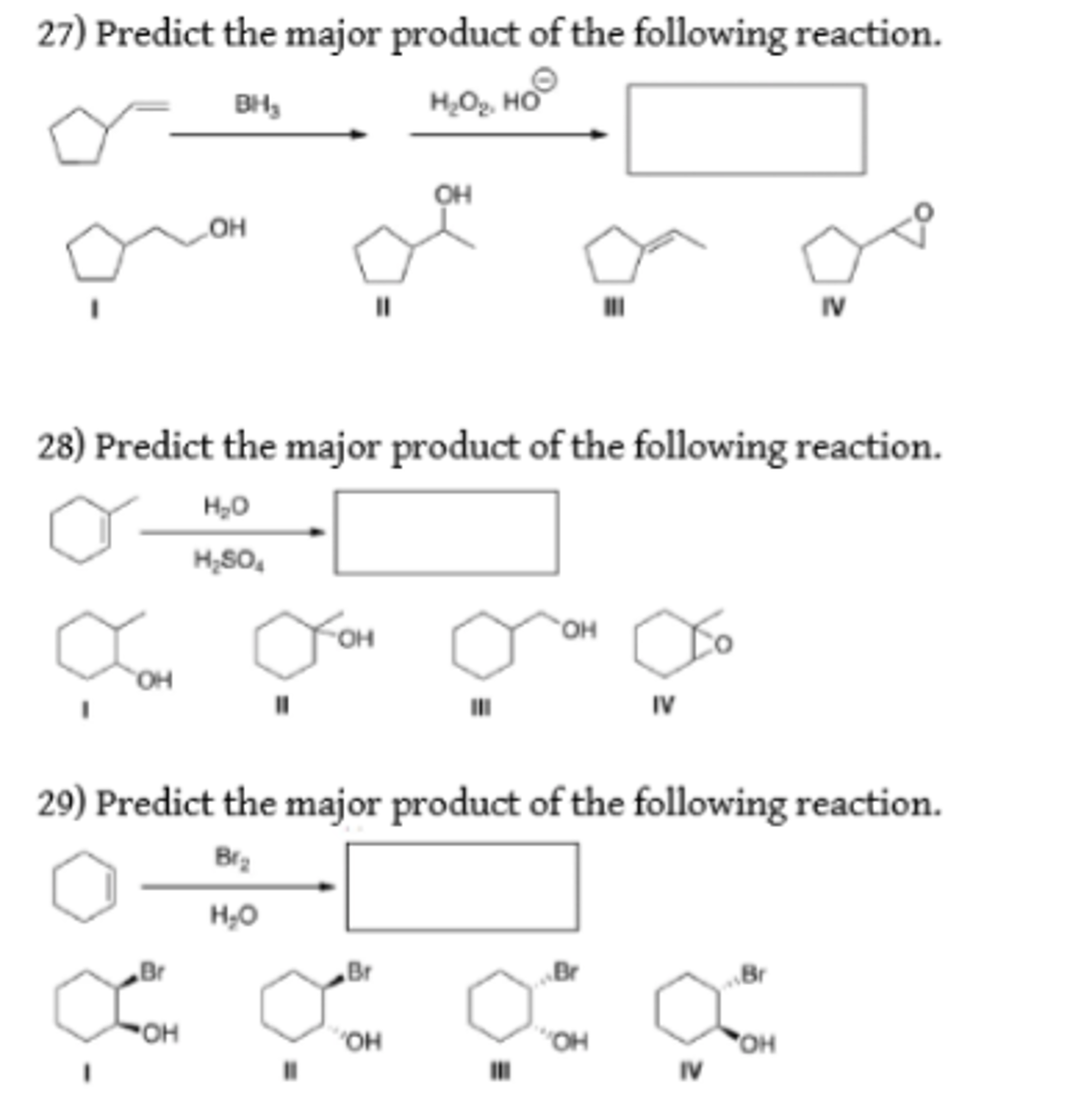
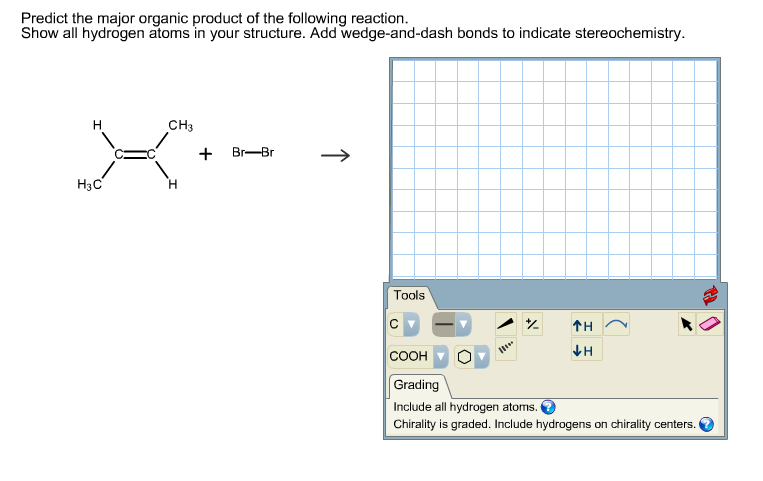
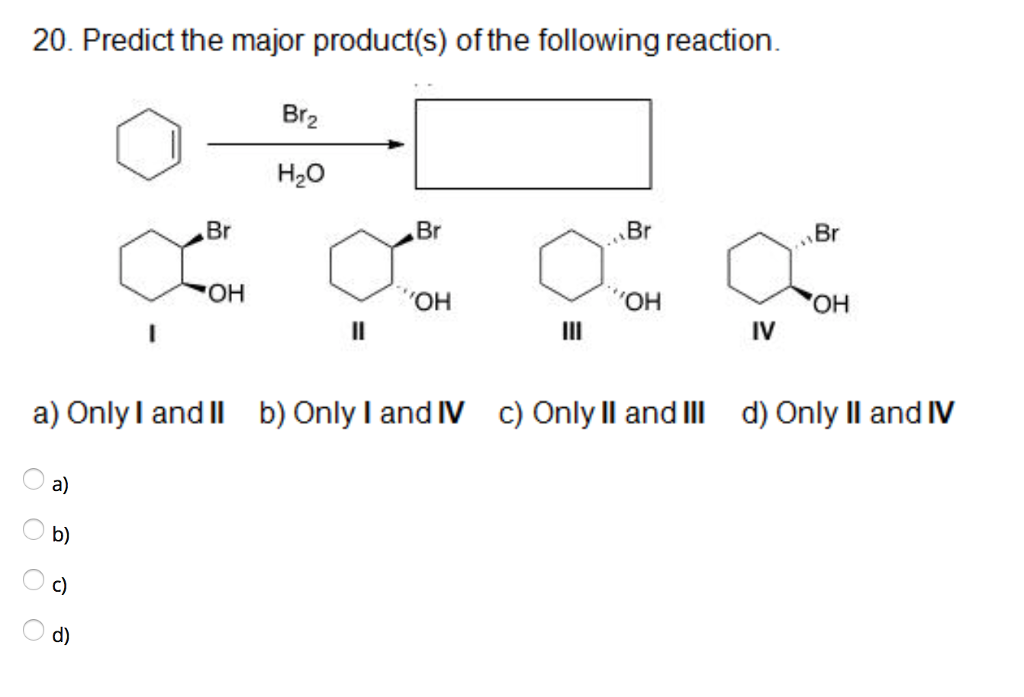
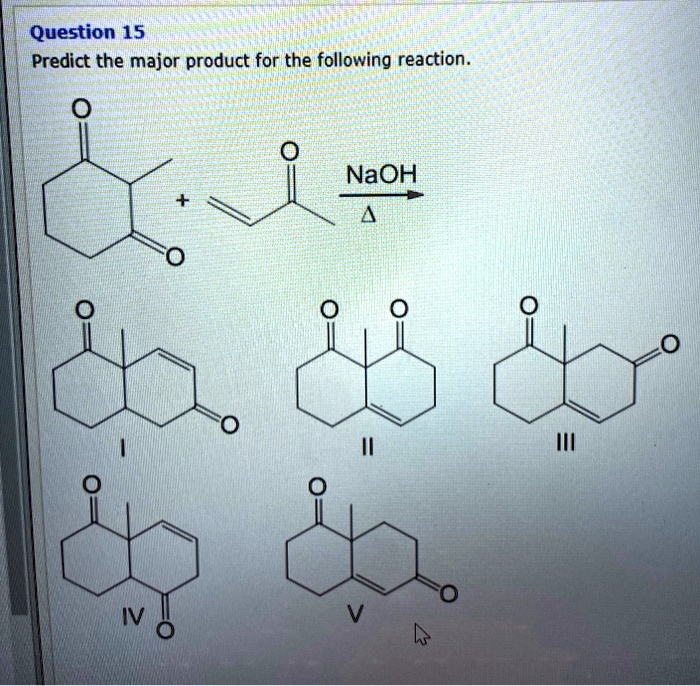
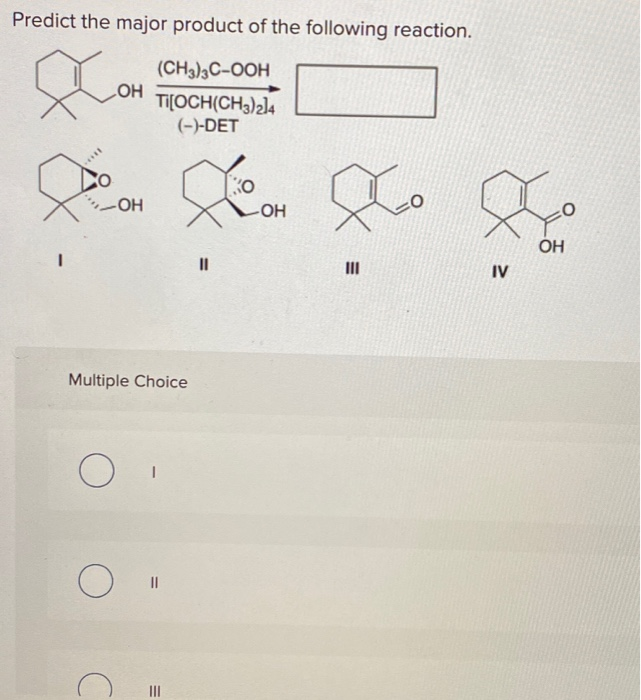
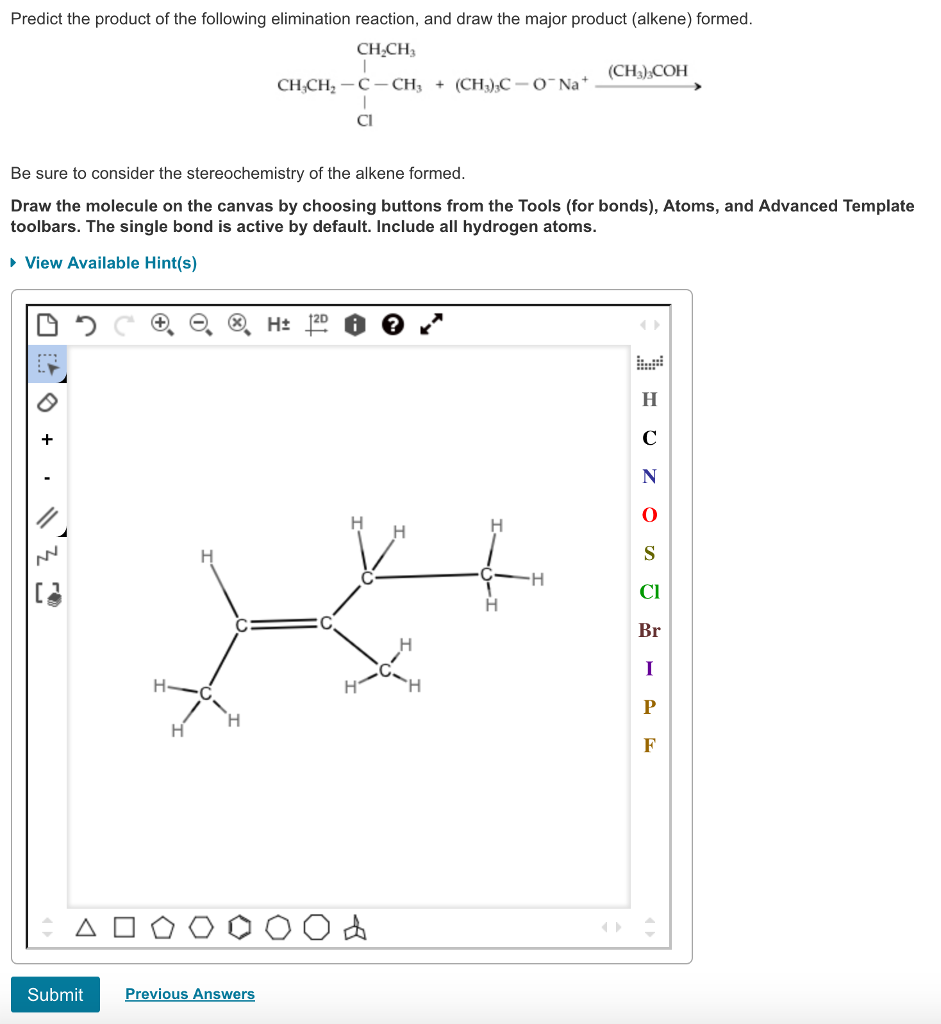
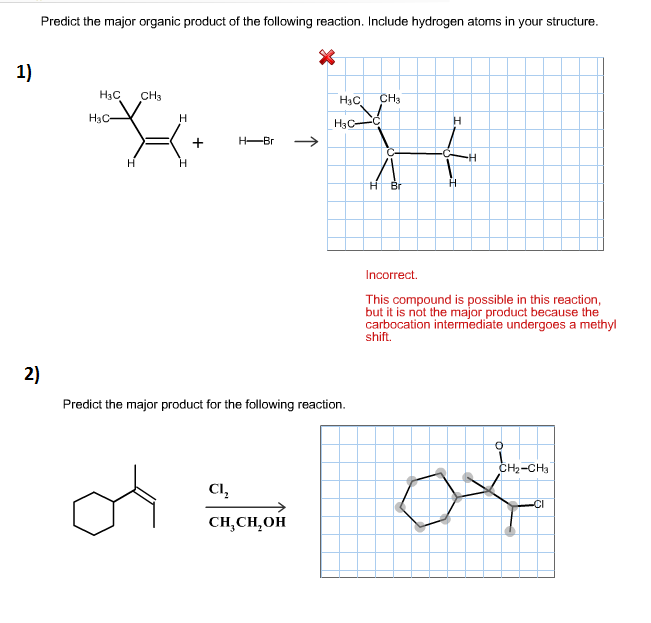
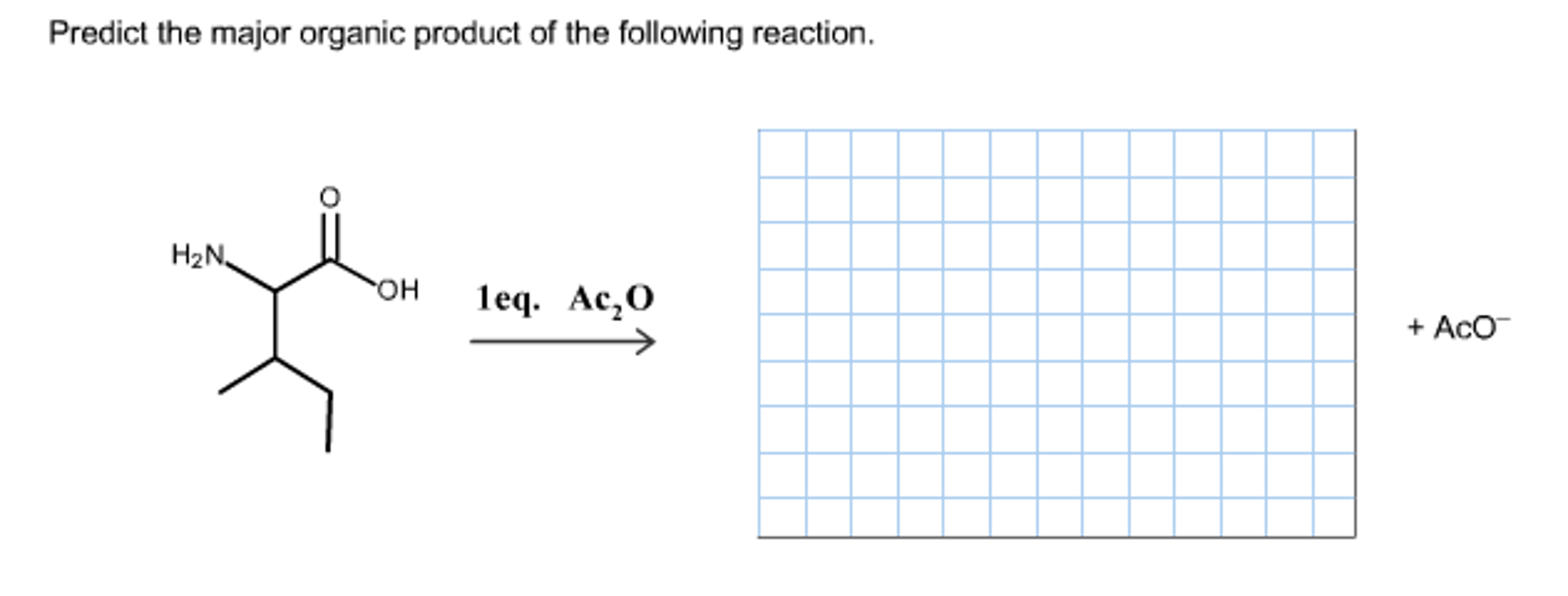
Organic chemistry reactions can appear daunting, but predicting the major product of a given reaction becomes manageable with a systematic approach. This article outlines the key principles and considerations involved in product prediction, enabling a better understanding of chemical transformations.
Understanding the Reaction Components
Before attempting to predict the product, it's crucial to thoroughly understand the reactants and reagents involved. The reactants are the starting materials that will undergo a chemical change. The reagents are the substances added to facilitate the reaction, often including catalysts, solvents, and other chemicals necessary for the transformation to occur. Analyzing the functional groups present in the reactants is particularly important, as they are the sites where chemical reactions will most likely take place.
Identifying the Substrate
Within the reactants, it's helpful to distinguish the substrate. The substrate is the molecule that undergoes the primary transformation. Identifying the substrate allows you to focus on the changes happening to that specific molecule.
Understanding the Reagent's Role
Reagents play a diverse role in organic reactions. Some reagents act as electrophiles, seeking electron-rich areas in the substrate. Others act as nucleophiles, donating electrons to electron-deficient areas. Some reagents are acids or bases, facilitating proton transfer. Still others are oxidizing or reducing agents, changing the oxidation state of the substrate.
Key Reaction Mechanisms
Predicting the major product frequently involves understanding the underlying reaction mechanism. A reaction mechanism is a step-by-step description of how a chemical reaction proceeds. While memorizing all mechanisms is impractical, familiarity with common mechanistic patterns can significantly aid product prediction.
SN1 and SN2 Reactions
SN1 (Substitution Nucleophilic Unimolecular) and SN2 (Substitution Nucleophilic Bimolecular) reactions are fundamental in organic chemistry. SN1 reactions proceed through a carbocation intermediate, favoring tertiary carbons and protic solvents. SN2 reactions occur in a single step, favoring primary carbons and aprotic solvents, with inversion of stereochemistry at the reaction center.
Elimination Reactions (E1 and E2)
Elimination reactions involve the removal of atoms or groups from a molecule, often forming a double bond. E1 (Elimination Unimolecular) reactions, like SN1, proceed through a carbocation intermediate and follow Zaitsev's rule (the most substituted alkene is favored). E2 (Elimination Bimolecular) reactions occur in a single step, requiring a strong base and often exhibiting stereochemical preferences (anti-periplanar geometry is favored).
Addition Reactions
Addition reactions involve the addition of atoms or groups to a molecule, typically across a multiple bond. Common examples include the addition of hydrogen halides (HX) to alkenes (following Markovnikov's rule: the hydrogen adds to the carbon with more hydrogens), the addition of water (hydration), and the addition of halogens.
Electrophilic Aromatic Substitution (EAS)
Electrophilic aromatic substitution involves the substitution of an atom (usually hydrogen) on an aromatic ring with an electrophile. The position of substitution is determined by the directing effects of substituents already present on the ring (ortho/para-directing or meta-directing, activating or deactivating).
Predicting the Major Product: A Step-by-Step Approach
Once you understand the reaction components and key mechanisms, you can follow a systematic approach to predict the major product.
- Identify the Functional Groups: Determine the functional groups present in the substrate. These are the reactive sites where changes will likely occur.
- Analyze the Reagents: Identify the reagents and their roles (electrophile, nucleophile, acid, base, oxidizing agent, reducing agent).
- Propose a Mechanism: Based on the reactants and reagents, propose a plausible reaction mechanism. This may involve multiple steps.
- Consider Regioselectivity: Regioselectivity refers to the preference for reaction to occur at one region of a molecule over another. Markovnikov's rule is an example of regioselectivity.
- Consider Stereoselectivity: Stereoselectivity refers to the preference for the formation of one stereoisomer over another. SN2 reactions exhibit stereospecificity (a type of stereoselectivity) where inversion of configuration occurs.
- Draw the Product(s): Based on the proposed mechanism, draw the possible products of the reaction.
- Determine the Major Product: Evaluate the factors that influence the stability and formation of the products. Consider steric hindrance, electronic effects, and the stability of intermediates.
Factors Influencing Product Distribution
Several factors can influence the distribution of products in a chemical reaction, leading to a major product and one or more minor products.
Steric Hindrance
Bulky groups can hinder the approach of reagents, affecting the rate of reaction and the regioselectivity. Reactions will often favor less sterically hindered sites.
Electronic Effects
Electronic effects, such as inductive and resonance effects, can influence the stability of intermediates and the reactivity of different sites in a molecule. Carbocation stability (tertiary > secondary > primary) is a crucial electronic effect to consider.
Leaving Group Ability
In substitution and elimination reactions, the leaving group's ability to depart significantly impacts the reaction rate. Good leaving groups are weak bases (e.g., halides, sulfonates).
Solvent Effects
The solvent can influence reaction rates and product distribution. Protic solvents (e.g., water, alcohols) can stabilize charged intermediates, favoring SN1 and E1 reactions. Aprotic solvents (e.g., DMSO, acetone) favor SN2 and E2 reactions by not solvating the nucleophile as strongly.
Examples and Practice
The best way to master product prediction is through practice. Working through numerous examples, analyzing the reactants, proposing mechanisms, and evaluating the factors that influence product distribution will greatly enhance your skills. Consult textbooks and online resources for practice problems and solutions.
For instance, consider the addition of HBr to propene (CH3CH=CH2). HBr is a strong acid that adds according to Markovnikov's rule. Therefore, the major product will be 2-bromopropane (CH3CHBrCH3), where the bromine atom adds to the more substituted carbon of the double bond.
In another example, consider the reaction of 2-bromobutane with a strong base like potassium tert-butoxide. This reaction favors an E2 elimination. Zaitsev's rule predicts that the major product will be the more substituted alkene, which in this case is 2-butene. However, due to steric hindrance of the bulky base, the Hofmann product (1-butene), the less substituted alkene may be formed as a minor product.
Conclusion
Predicting the major product of an organic reaction is a fundamental skill in organic chemistry. By understanding the reactants, reagents, and reaction mechanisms, and by systematically considering factors such as steric hindrance, electronic effects, and solvent effects, one can effectively predict the major product. This ability is crucial for designing synthetic routes, understanding reaction outcomes, and advancing our knowledge of chemical transformations.