The fundamental building block of all matter is the atom. While atoms share common components, their properties and behaviors vary significantly. Understanding what defines the identity of an atom is crucial for comprehending the vast diversity of elements and their interactions.
The Defining Role of the Proton
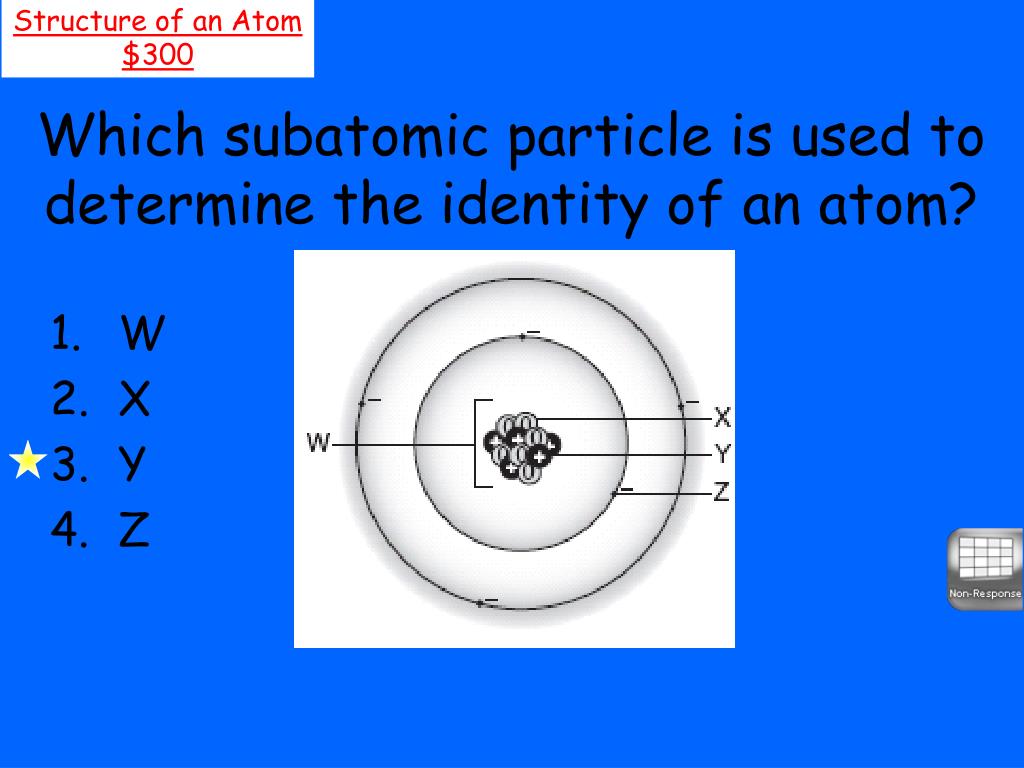
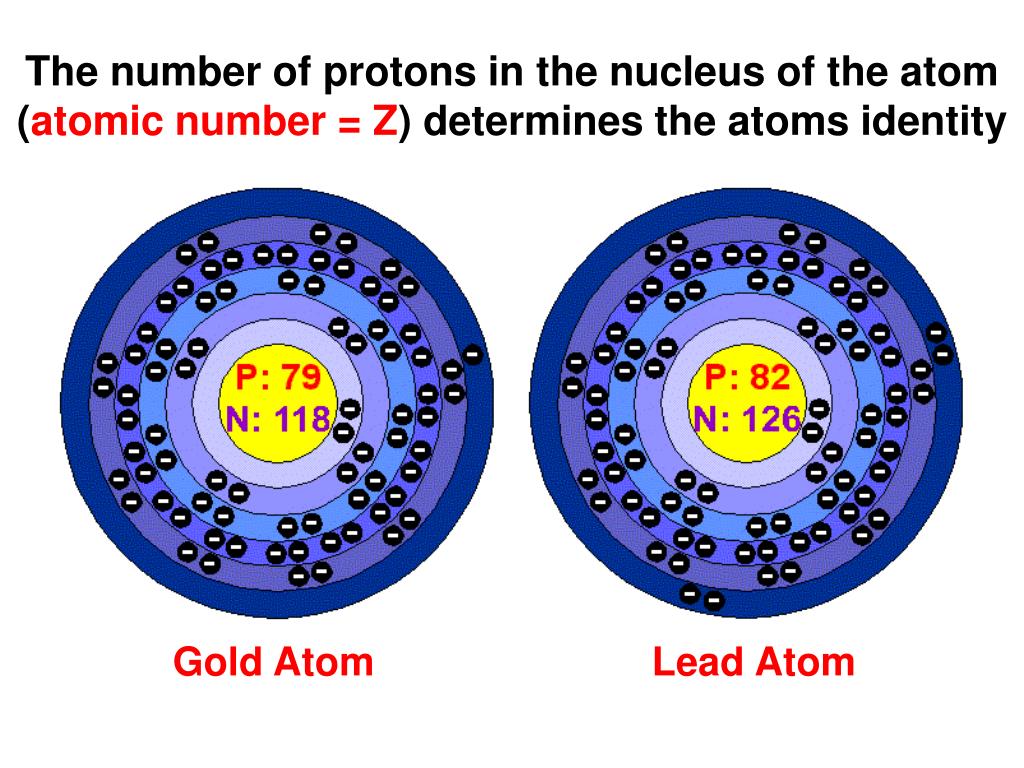
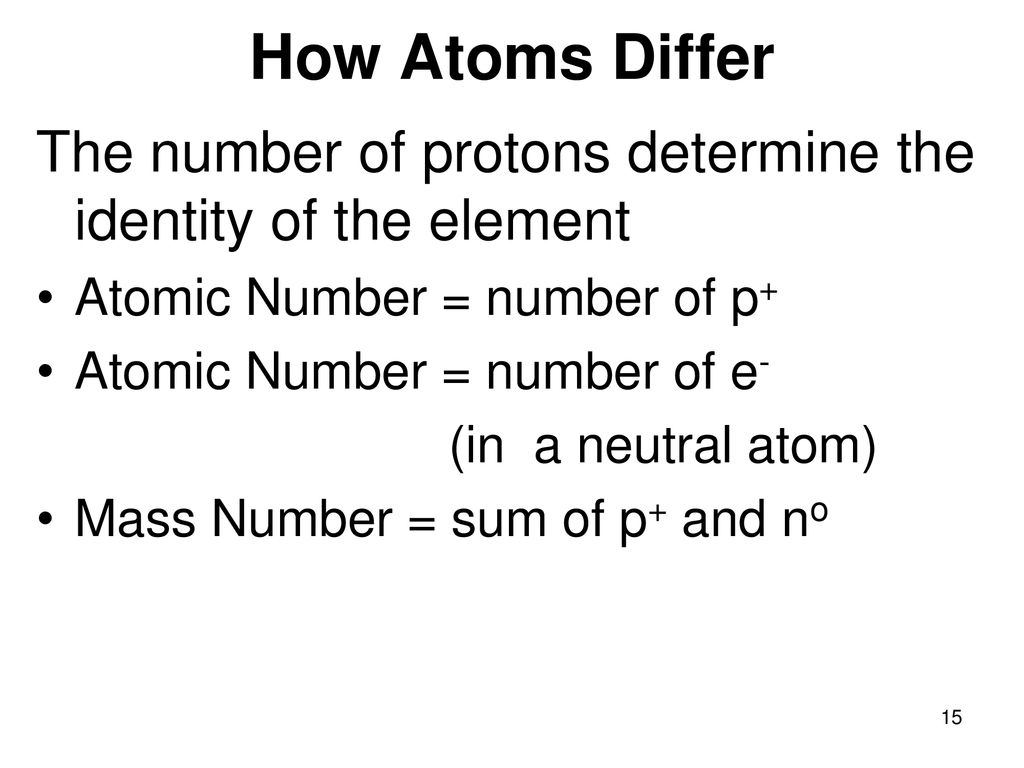
The identity of an atom is unequivocally determined by the number of protons it contains. Protons are positively charged particles located within the atom's nucleus. This number is known as the atomic number (Z), and it is a unique identifier for each element. Every element on the periodic table is defined by a specific atomic number. For instance, all atoms with one proton are hydrogen atoms (Z=1), all atoms with six protons are carbon atoms (Z=6), and all atoms with 79 protons are gold atoms (Z=79).
Changing the number of protons in an atom fundamentally changes the element itself. If you were to somehow add a proton to a carbon atom (Z=6), it would no longer be carbon; it would become a nitrogen atom (Z=7). This highlights the critical and defining role of protons in determining the identity of an atom.
The atomic number, representing the number of protons, is the fingerprint of an element.
Why Protons Define Elemental Identity
The importance of protons stems from their direct influence on the atom's electronic structure and, consequently, its chemical behavior. The number of protons dictates the positive charge of the nucleus. In a neutral atom, this positive charge is balanced by an equal number of negatively charged electrons orbiting the nucleus. The arrangement of these electrons, also known as the electron configuration, determines how an atom interacts with other atoms to form chemical bonds.
Consider the example of sodium (Na), which has an atomic number of 11. A neutral sodium atom has 11 protons in its nucleus and 11 electrons surrounding the nucleus. These electrons are arranged in specific energy levels or shells. Sodium readily loses one electron to achieve a more stable electron configuration. This tendency to lose an electron defines sodium's chemical reactivity and its ability to form ionic bonds with elements like chlorine (Cl).
If the number of protons in sodium were to change, the electron configuration would also change, leading to vastly different chemical properties. Thus, the number of protons directly influences the electron configuration and, therefore, the chemical identity of the element.
The Influence of Neutrons and Isotopes
While the number of protons defines the element, the number of neutrons can vary within atoms of the same element. Neutrons are neutral particles also located in the nucleus. Atoms of the same element that have different numbers of neutrons are called isotopes.
For example, carbon (Z=6) exists in several isotopic forms. The most common isotope is carbon-12 (12C), which has 6 protons and 6 neutrons. However, carbon also exists as carbon-13 (13C), with 6 protons and 7 neutrons, and carbon-14 (14C), with 6 protons and 8 neutrons.
Isotopes of an element share nearly identical chemical properties because they have the same number of protons and, therefore, the same electron configuration. However, isotopes differ in their mass due to the varying number of neutrons. This mass difference can lead to subtle differences in physical properties, such as density and reaction rates.
Radioactive Isotopes
Some isotopes are unstable, meaning their nuclei spontaneously decay, emitting particles and energy. These are known as radioactive isotopes or radioisotopes. Carbon-14 (14C) is a well-known example of a radioisotope. It decays over time, and this decay is used in radiocarbon dating to determine the age of ancient organic materials.
The different isotopes of an element do not change the fundamental identity of that element. Carbon-12, carbon-13, and carbon-14 are all still carbon because they all have 6 protons. The differing number of neutrons only affects the mass and stability of the nucleus, but not the chemical properties determined by the electron configuration.
The Role of Electrons and Ions
Electrons are negatively charged particles that orbit the nucleus in specific energy levels or shells. In a neutral atom, the number of electrons is equal to the number of protons, resulting in a net charge of zero. However, atoms can gain or lose electrons to form ions.
If an atom loses electrons, it becomes a positively charged ion called a cation. For example, if a sodium atom (Na) loses one electron, it becomes a sodium ion (Na+). If an atom gains electrons, it becomes a negatively charged ion called an anion. For example, if a chlorine atom (Cl) gains one electron, it becomes a chloride ion (Cl-).
While the number of electrons influences the charge of an atom and its ability to form chemical bonds, it does not change the fundamental identity of the element. A sodium ion (Na+) is still sodium, and a chloride ion (Cl-) is still chlorine. The number of protons remains constant, defining the element's identity.
Ions exhibit different chemical behavior compared to their neutral counterparts. For instance, sodium metal (Na) is a highly reactive element that reacts violently with water. However, sodium ions (Na+) are stable in water and are essential components of many biological systems.
Summary and Significance
In summary, the identity of an atom is determined by the number of protons in its nucleus, also known as the atomic number. The atomic number is a unique identifier for each element and dictates the atom's electron configuration and, therefore, its chemical properties. While the number of neutrons can vary, leading to isotopes, and the number of electrons can change, resulting in ions, these variations do not alter the fundamental identity of the element, which is defined by its proton number.
Understanding what defines the identity of an atom is essential for several reasons:
*Comprehending the Periodic Table: The periodic table is organized based on the atomic number, providing a systematic way to understand the properties and relationships of different elements.
*Predicting Chemical Behavior: Knowing the atomic number allows us to predict how an element will interact with other elements to form chemical compounds.
*Understanding Nuclear Processes: The number of protons and neutrons in the nucleus determines the stability of an atom and its ability to undergo nuclear reactions.
*Advancements in Various Fields: This understanding is crucial in fields like chemistry, physics, materials science, medicine, and environmental science.
Ultimately, the simple concept that the number of protons defines an atom's identity unlocks a profound understanding of the world around us, from the smallest molecules to the largest stars.
.jpg)














+is+the+number+of+protons%3B+this+determines+the+identity+of+the+element..jpg)






