Hey there, fellow knowledge adventurers! Ever wonder how your body wages war against nasty invaders like bacteria and viruses? It's a pretty epic story, and a key part of that story involves some seriously cool molecules called antibodies. And guess what? We're diving into the nitty-gritty of these molecular warriors today, focusing on their *variable* and *constant* regions. Trust me, this isn't just some boring biology lesson; it's about understanding the amazing machinery that keeps you healthy and kicking!
Antibodies: Your Body's Elite Special Forces
Think of antibodies as your body's elite special forces. They're proteins produced by your immune system, specifically by B cells, and their sole mission is to seek out and destroy foreign invaders, also known as antigens. These antigens can be anything from viruses and bacteria to fungi and even toxins.
Now, here's the cool part. Each antibody is designed to recognize and bind to a *specific* antigen, like a lock and key. This specificity is what makes them so effective. But how do they achieve this incredible precision? That's where the variable and constant regions come into play!
Decoding the Antibody Structure
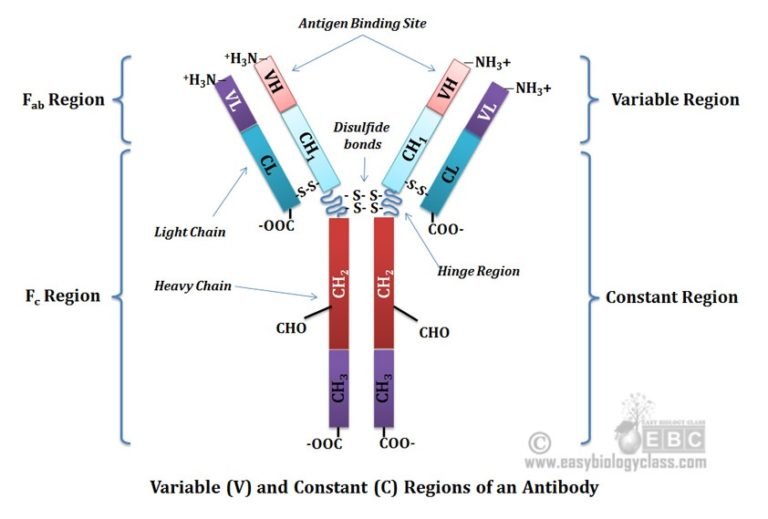
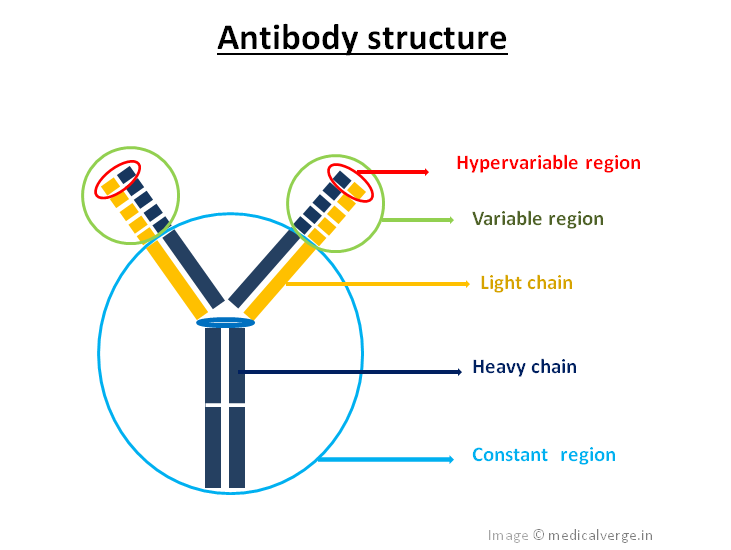
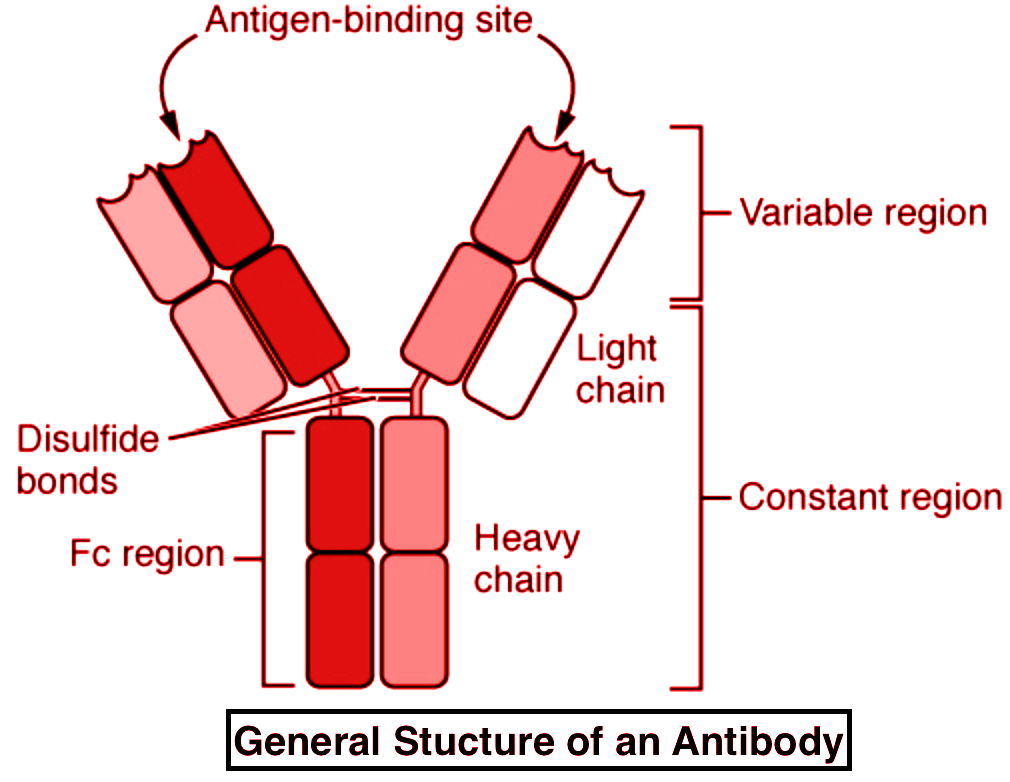
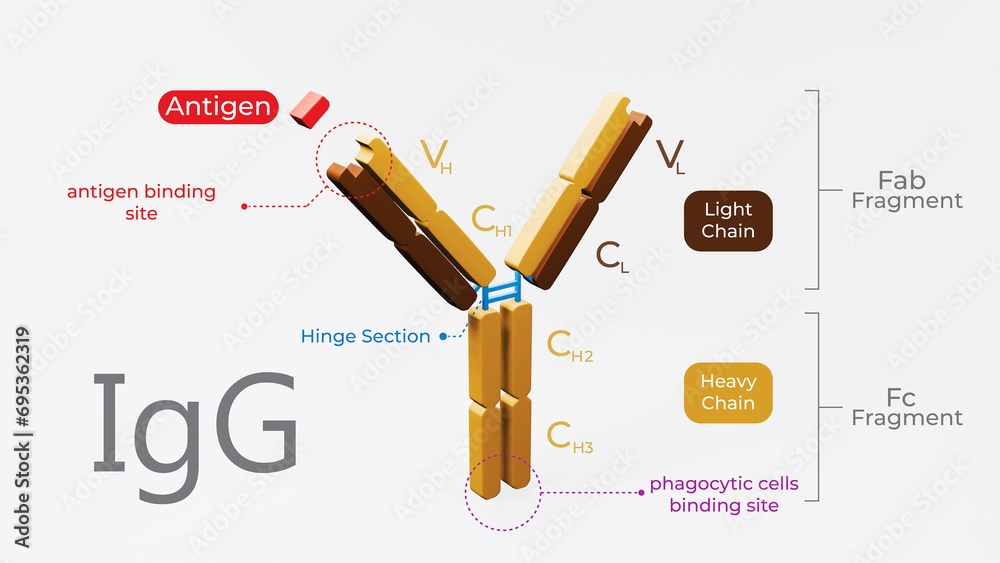
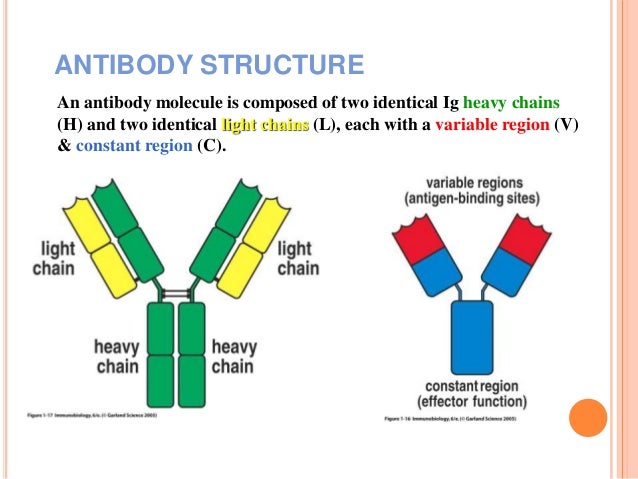
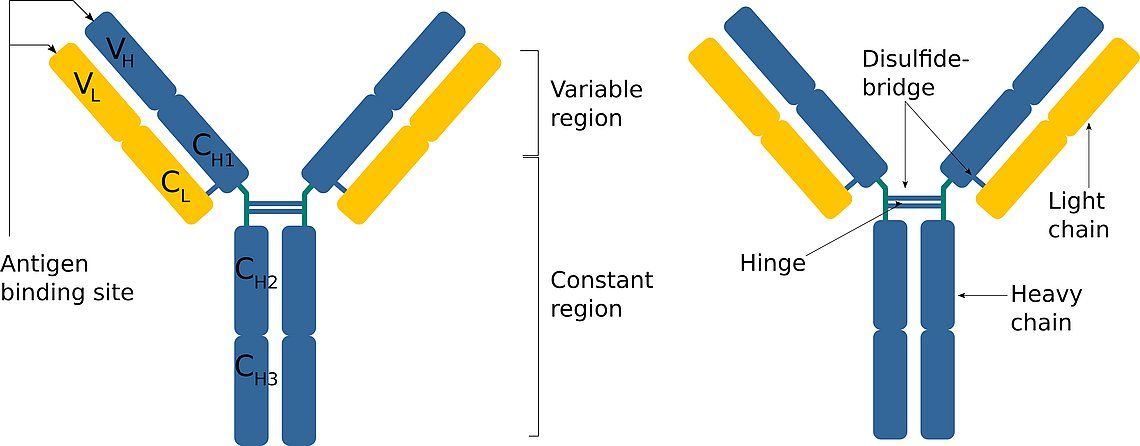
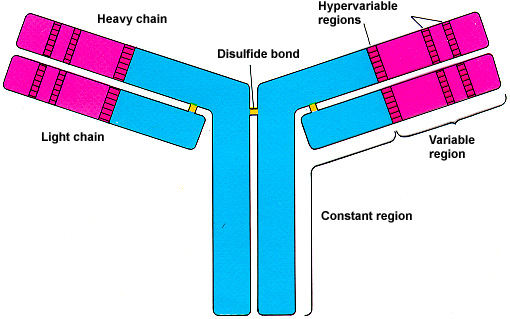
Imagine an antibody as a "Y" shaped molecule. It's made up of two identical heavy chains and two identical light chains, all linked together by disulfide bonds (think of them as tiny molecular rivets). Okay, I know it sounds a bit technical, but stay with me!
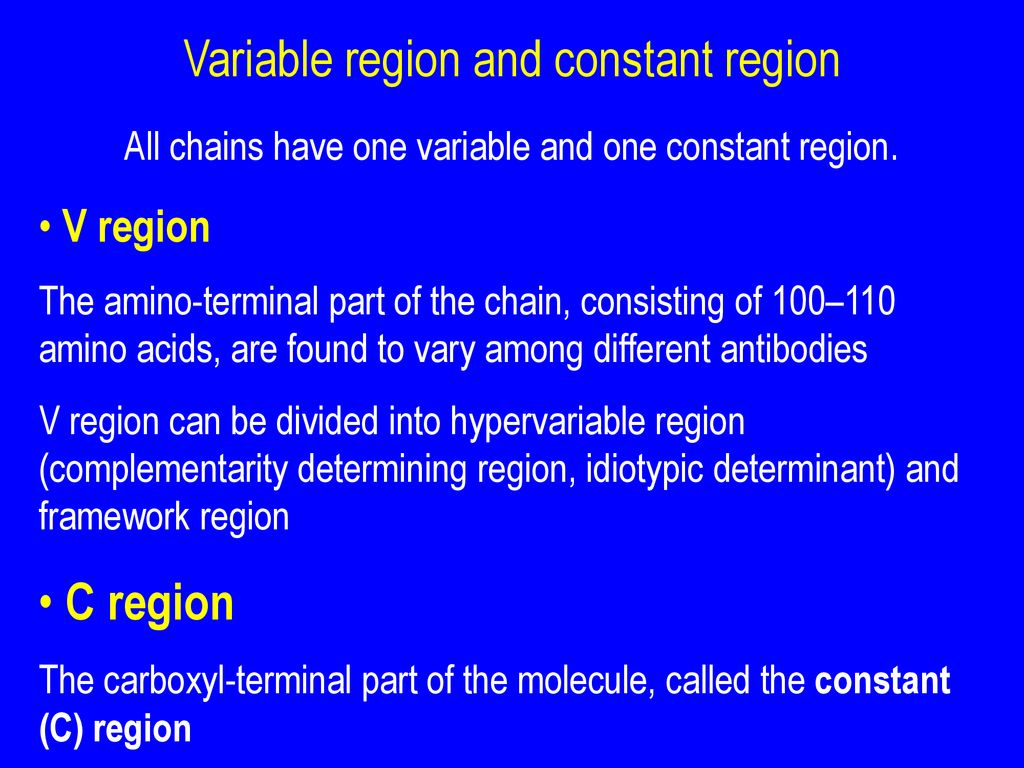
Each chain, both heavy and light, has a *variable* region and a *constant* region. It's like a modular design – smart, right? Let’s break it down:
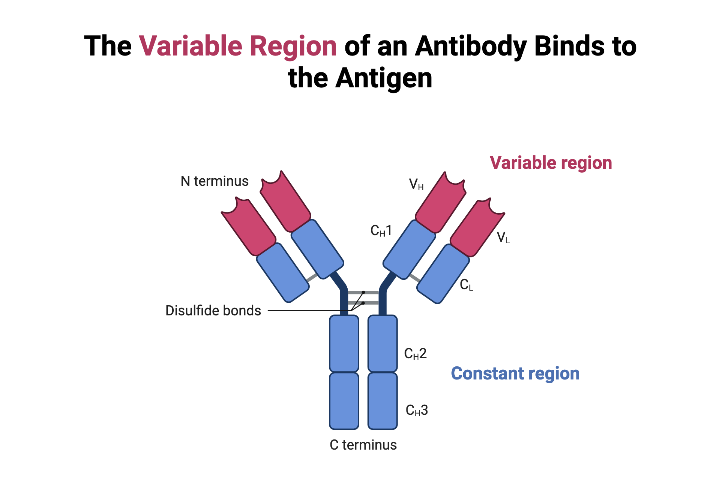
- Variable Region (V Region): This is the antigen-binding site, the "lock-picking" part of the antibody. It's located at the tips of the "Y" arms.
- Constant Region (C Region): This region forms the stem of the "Y" and is responsible for the antibody's effector functions, like activating other immune cells or triggering complement activation (a cascade of protein reactions that helps destroy pathogens).
See? Not so scary after all! Now, let's zoom in on each of these regions and see what makes them so special.
The Variable Region: Where the Magic Happens
Alright, let's get to the heart of the matter: the variable region. This is the most crucial part of the antibody because it determines what antigen the antibody can bind to.
Each antibody has two identical variable regions, one on each arm of the "Y." These regions are formed by the variable domains of both the heavy and light chains. Within the variable region are even more specialized areas called hypervariable regions or complementarity-determining regions (CDRs). These are the *most* variable parts of the antibody and are directly responsible for contacting the antigen.
Think of it like this: the variable region is a glove, and the hypervariable regions are the fingerprints on that glove. The shape and arrangement of amino acids in these hypervariable regions create a unique binding surface that perfectly matches a specific antigen.
The diversity of these variable regions is absolutely mind-boggling. Your body can generate an almost limitless number of different antibodies, each with a unique variable region capable of recognizing a different antigen. How does it do this? Through a clever process called V(D)J recombination (more on that another time – it's a whole other fascinating rabbit hole!).
So, in a nutshell, the variable region is the key to the antibody's specificity. It's the part that allows the antibody to recognize and bind to its target antigen with incredible precision. Pretty neat, huh?
The Constant Region: The Antibody's Swiss Army Knife
Now, let's turn our attention to the constant region. While the variable region is all about specificity, the constant region is all about *function*. It's the part of the antibody that determines what the antibody *does* after it binds to an antigen.
The constant region is located on the stem of the "Y" and is much less variable than the variable region (hence the name "constant"). It's made up of constant domains of the heavy chains (and sometimes the light chains, depending on the antibody type).
There are different classes of antibodies, also known as isotypes (IgG, IgM, IgA, IgE, IgD), each with a different constant region. These different constant regions determine the antibody's effector functions, such as:
- Activating complement: Some constant regions can trigger the complement system, a cascade of proteins that leads to the destruction of pathogens.
- Recruiting immune cells: Constant regions can bind to receptors on immune cells, such as macrophages and neutrophils, signaling them to engulf and destroy the antigen-antibody complex.
- Neutralizing toxins: Some antibodies can bind to toxins and prevent them from interacting with their target cells.
- Triggering allergic reactions: IgE antibodies, for example, bind to mast cells and basophils, triggering the release of histamine and other inflammatory mediators, leading to allergic reactions.
- Antibody-dependent cell-mediated cytotoxicity (ADCC): Antibodies can coat infected cells and then bind to NK cells (natural killer cells) via their constant region. This triggers the NK cell to release cytotoxic granules and kill the infected cell.
So, the constant region is like the antibody's Swiss Army knife. It equips the antibody with a range of tools to fight off infection. The specific constant region determines which tools the antibody will use.
Variable vs. Constant: A Side-by-Side Comparison
To really solidify your understanding, let's compare and contrast the variable and constant regions:
| Feature | Variable Region | Constant Region |
|---|---|---|
| Variability | Highly variable | Relatively constant |
| Location | Tips of the "Y" arms | Stem of the "Y" |
| Function | Antigen binding (specificity) | Effector functions (e.g., complement activation, immune cell recruitment) |
| Composition | Variable domains of heavy and light chains (includes hypervariable regions) | Constant domains of heavy chains (and sometimes light chains) |
| Diversity | Enormous diversity (generated by V(D)J recombination) | Limited diversity (defines antibody isotype) |
As you can see, these two regions work together in perfect harmony to ensure that antibodies can both recognize and eliminate threats. It's a true masterpiece of molecular engineering!
Why Should You Care About Antibody Regions?
Okay, so you might be thinking, "This is all fascinating, but why should I care about the variable and constant regions of antibodies?"
Well, here's the thing: understanding antibody structure and function is crucial for developing new and improved treatments for a wide range of diseases.
For example:
- Monoclonal antibodies: These are antibodies produced by a single clone of B cells, meaning they all have the same variable region and bind to the same antigen. They're used to treat a variety of diseases, including cancer, autoimmune disorders, and infectious diseases. Drugs like Humira (adalimumab) and Rituxan (rituximab) are monoclonal antibodies. Understanding the variable region allows scientists to design antibodies that specifically target cancer cells or other disease-causing agents.
- Vaccines: Vaccines work by stimulating your immune system to produce antibodies against a specific pathogen. Understanding the variable region helps scientists design vaccines that elicit a strong and long-lasting antibody response.
- Diagnostics: Antibodies are used in a variety of diagnostic tests, such as ELISA and Western blotting, to detect the presence of specific antigens in a sample. Understanding the variable region is essential for developing highly sensitive and specific diagnostic tests.
Moreover, antibody research is an ever-evolving field. Scientists are constantly discovering new ways to manipulate antibodies to improve their efficacy and specificity. Who knows, maybe *you* will be the one to make the next big breakthrough in antibody-based therapies!
The Future is Bright (and Full of Antibodies!)
So, there you have it! A whirlwind tour of the variable and constant regions of antibodies. I hope you found it as fascinating as I do.
Understanding the intricate details of these molecular warriors is not just about memorizing biology facts; it's about appreciating the incredible complexity and elegance of the human immune system. It's about realizing the potential for scientific innovation to improve human health. And it's about opening your mind to the wonders of the microscopic world that constantly works to keep us alive and healthy.
The world of immunology is constantly evolving, with new discoveries being made every day. So, if you're feeling inspired, I encourage you to delve deeper into this fascinating field. There are countless resources available online and in libraries. Who knows, maybe you'll even discover a new passion for science!
Keep exploring, keep questioning, and keep learning. The world is full of amazing things just waiting to be discovered. And remember, your body is a battlefield, and antibodies are your elite special forces, tirelessly working to protect you every single day. Now that's something to be thankful for!