Ever wonder about the big guys on the periodic table? You know, those rows and columns of elements that look like some sort of alien alphabet? Well, it turns out some of those letters (aka elements) are simply bigger than others. And when it comes to sheer size, measured by something called the atomic radius, one reigns supreme. So, let's dive in and find out who the heavyweight champion of the atomic world is!
Atomic Radius: Think of it as Atomic Belly Size!
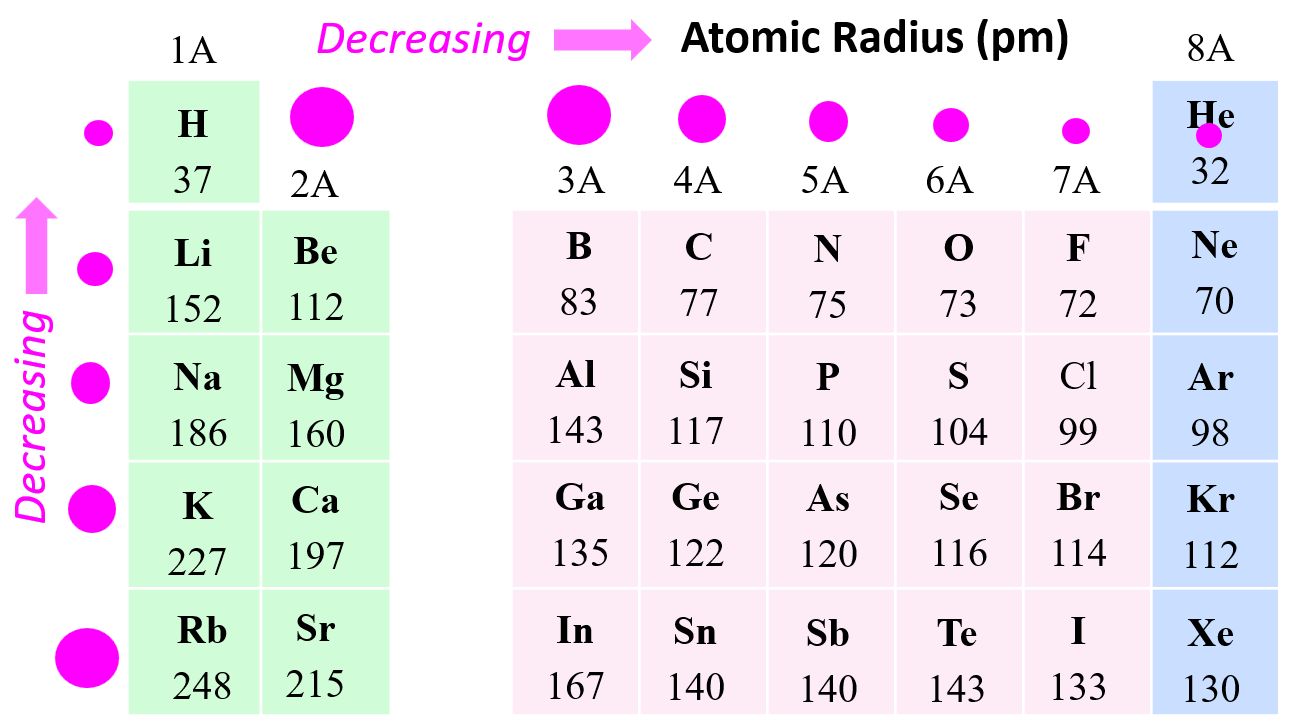
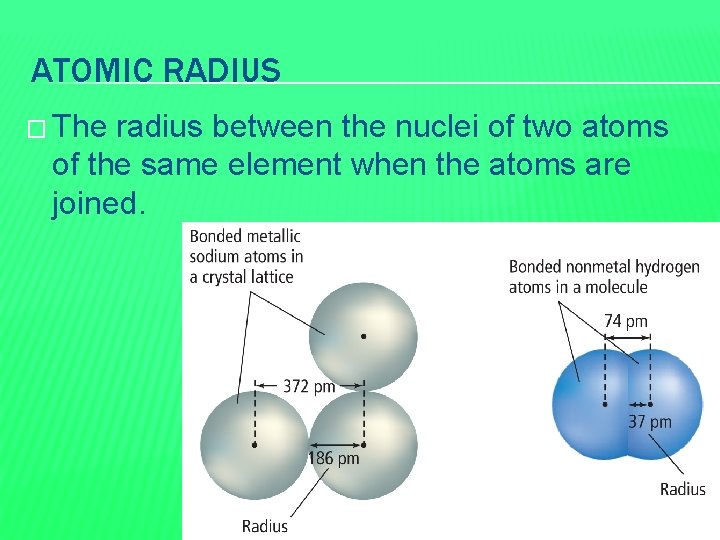
First things first, what exactly *is* atomic radius? Imagine each atom as a tiny, fuzzy sphere. The atomic radius is basically the distance from the center of that sphere (the nucleus, jam-packed with protons and neutrons) to its outer edge, where you’re most likely to find its electrons. Think of it like measuring the belly of an atom! Some atoms have a bigger “belly” because their electrons are spread out further, making them larger overall.
Now, the funny thing about atomic radius is that it isn't always straightforward to measure. Atoms are constantly buzzing and their electrons are always moving. It's like trying to measure the exact wingspan of a hummingbird – tough to pin down! But scientists have developed clever ways to estimate the average distance of the outermost electrons, giving us a reliable value for the atomic radius.
The Periodic Table: A Size Chart for Atoms
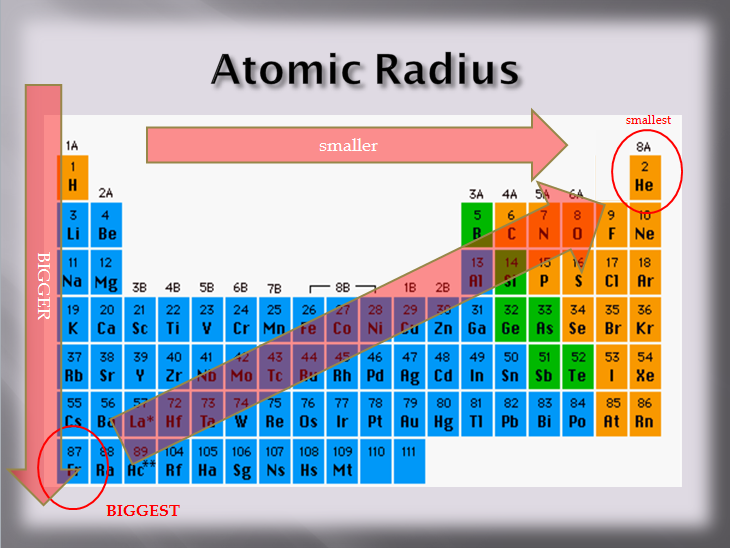
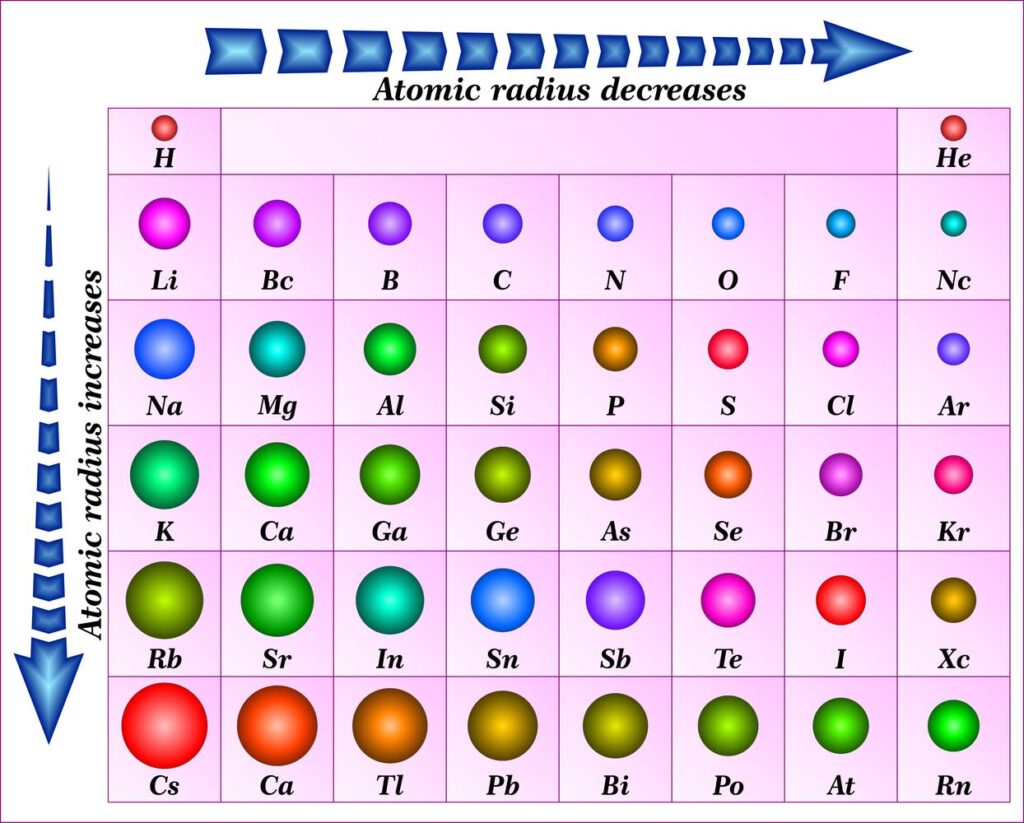
The periodic table isn't just a pretty chart; it's actually a carefully organized system where elements are arranged according to their properties, including atomic size. The cool thing is that atomic radius follows predictable trends as you move across and down the periodic table.
Here's the gist:
- Moving from left to right across a row (a "period"): Atomic radius generally *decreases*. Why? Because as you move across, the number of protons in the nucleus increases, creating a stronger positive charge. This stronger charge pulls the electrons in closer, shrinking the atom. Imagine trying to squeeze a stress ball harder – it gets smaller!
- Moving down a column (a "group"): Atomic radius generally *increases*. This is because as you move down, you're adding more electron shells. Each new shell is like adding another layer of clothing – the atom gets bigger and bulkier.
Think of it like this: the periodic table is like a seating chart at a really, *really* weird party. As you go across the row, the guests are getting squeezed closer together. As you go down a row, each guest has more layers of clothing making them bigger!
And The Winner Is... Francium!
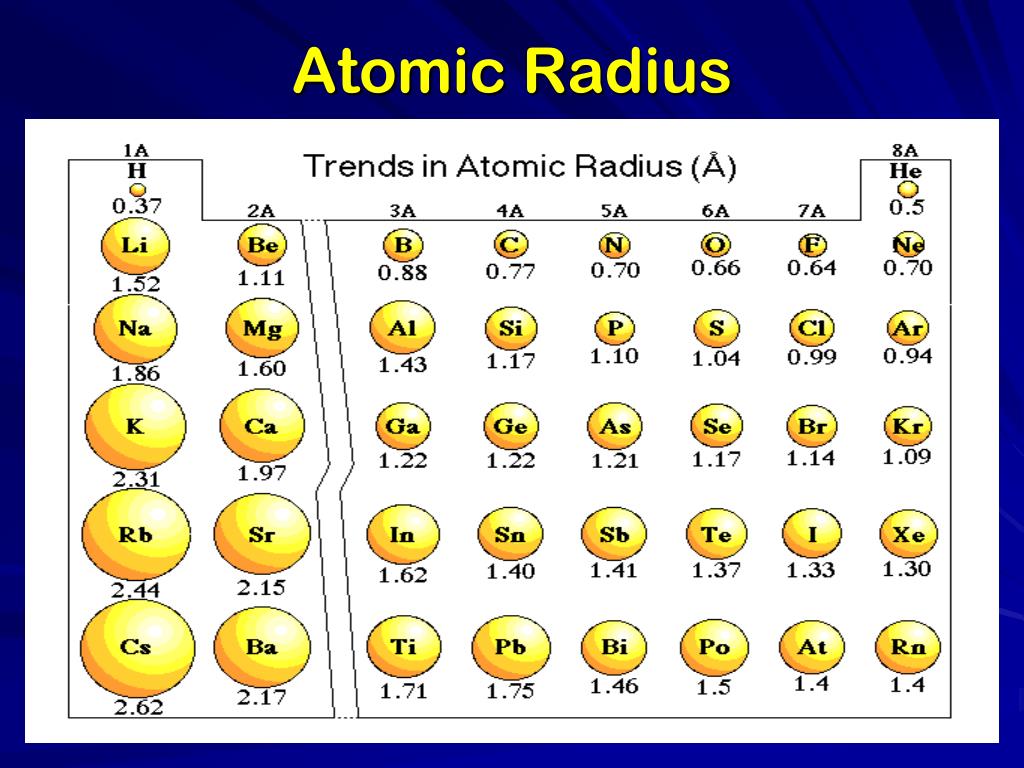
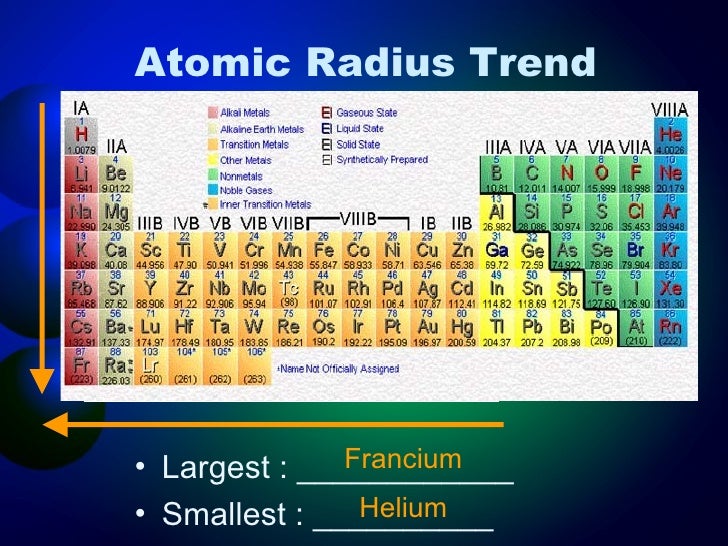
Okay, drumroll please... The atom with the largest atomic radius is Francium (Fr)! Located at the bottom left of the periodic table, Francium sits in the alkali metal group and boasts a whopping atomic radius of approximately 260 picometers (a picometer is a trillionth of a meter – seriously tiny!). That's huge... atomically speaking, of course!
Why Francium? Well, it's all about its position on the periodic table. It’s at the very bottom of its group (Group 1, the alkali metals), meaning it has the most electron shells. And it’s located on the left side, minimizing the squeezing effect of increasing nuclear charge.
You might be thinking, “Hold on, I thought Cesium (Cs) was the biggest!” And that's a fair point. For a long time, Cesium was considered the largest naturally occurring atom. Francium is actually radioactive and extremely rare, so accurately measuring its atomic radius is a challenge. Plus, because it decays so quickly, it's not used in many practical applications. So, while Cesium might be more practical to talk about, technically, Francium takes the crown. Think of it like this: Cesium is the champion boxer who's always in the ring, while Francium is the reclusive heavyweight champion who stays hidden in the mountains!
Why Should You Care About Atomic Radius?
Okay, so Francium is big. But why should you care? It's not like you're going to encounter Francium on your way to the grocery store (unless you happen to be shopping for very rare radioactive elements). The truth is, understanding atomic radius helps us understand the properties of materials all around us.
Here are a few reasons why atomic radius matters:
- Reactivity: The size of an atom influences how easily it can react with other atoms. Larger atoms tend to lose electrons more easily, making them more reactive. This is because the outer electrons are further away from the nucleus and less tightly held. Think of it like this: It's easier to pull a balloon away from someone if they are holding it with a long string (large radius) than a short one (small radius).
- Melting and Boiling Points: The strength of the metallic bonds in metals depends on the size of the atoms. Smaller atoms can pack together more tightly, leading to stronger bonds and higher melting and boiling points. Imagine trying to stack basketballs versus golf balls. The golf balls will fit together much more tightly and be harder to pull apart!
- Density: Atomic radius plays a role in determining the density of a substance. Denser materials have atoms that are packed tightly together.
- Material Properties: From the strength of steel to the conductivity of copper, atomic radius is a key factor in determining the properties of all sorts of materials that we use every day.
For example, the alkali metals (like Francium and Cesium) are highly reactive because they have large atomic radii, making it easy for them to lose electrons and form chemical bonds. This is why they're never found in their pure form in nature; they're always bonded to other elements.
Francium: The Unreachable Giant
While you're unlikely to ever handle Francium, knowing about its gigantic atomic size helps to illustrate fundamental principles about the behavior of atoms and their role in the world around us. It highlights the trends in the periodic table and emphasizes how atomic size influences a whole host of chemical and physical properties.
So, next time you look at the periodic table, remember Francium, the unreachable giant, and appreciate how something so tiny can have such a significant impact on the world of materials.
Think of Francium as the Andre the Giant of the atomic world - big, rare, and someone you wouldn't want to mess with!
Now, go forth and impress your friends with your newfound knowledge of atomic radii!