Ever squeeze a lemon into your tea and watch it change color? Or maybe you’ve accidentally splashed vinegar while making salad dressing and puckered up? That's acid at work, my friend, and understanding what happens when acids meet water is simpler than you think. Think of it like this: acids are the drama queens (or kings!) of the chemistry world, and water is their stage.
The Great Acid Unveiling: What Happens in Water?
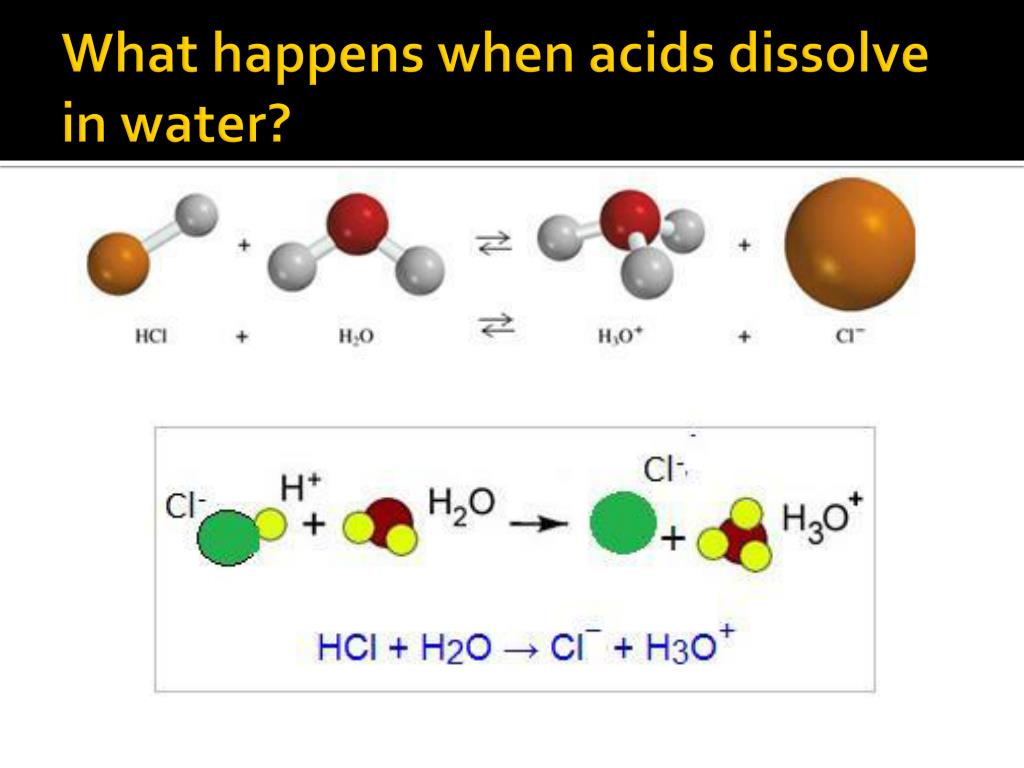
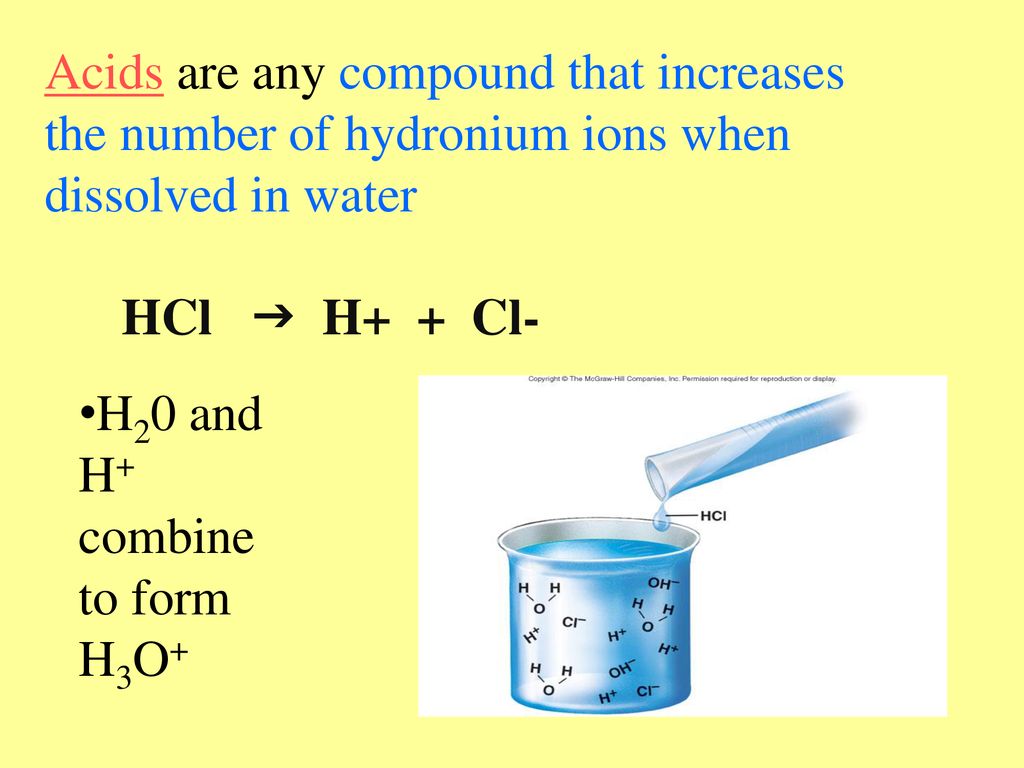
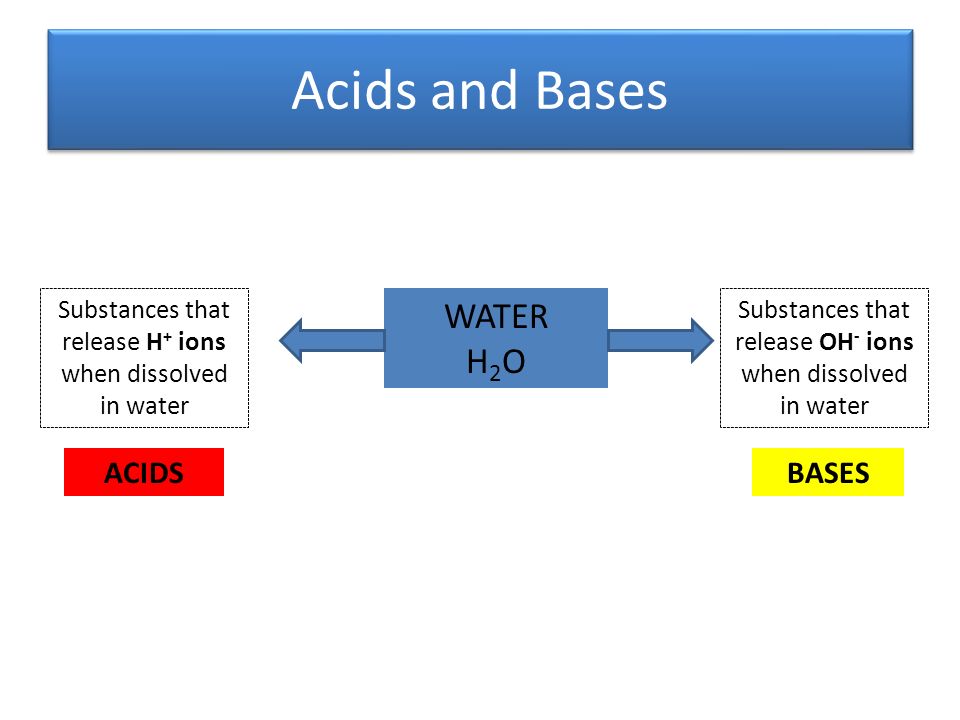
So, what *actually* happens when you dissolve an acid in water? The short answer: it releases hydrogen ions (H+). But let's unpack that a little, because "hydrogen ions" sounds like something out of a sci-fi movie, not your everyday kitchen chemistry.
Think of an acid molecule like a couple holding hands really tight. In this case, it's a hydrogen atom (H) holding hands with something else (we'll call it "X"). When water comes along, it's like that charming third wheel who whispers sweet nothings and tempts the hydrogen to leave its partner.
The water molecules are attracted to both the hydrogen and the "X," and because water is a super-solvent (meaning it's really good at pulling things apart), it manages to coax the hydrogen away. This newly single hydrogen atom becomes a hydrogen ion (H+) – a positively charged particle just floating around. The "X" is now on its own too, usually as a negatively charged ion.
Basically, acids are generous souls. When they're dissolved in water, they give away H+ ions like Oprah giving away cars (but instead of cars, it's... well, tiny charged particles). The more H+ ions floating around, the more acidic the solution becomes. Imagine a pool party where the more people you cram in the pool, the more crowded and chaotic it gets – same principle!
Strong vs. Weak Acids: The Intensity Factor
Now, not all acids are created equal. Some are the shy types who barely release any H+ ions, while others are the party animals who ditch their "X" partner the second water shows up. This leads us to the concept of strong and weak acids.
Strong acids are like those friends who are always up for anything. They completely dissociate (split apart) in water, meaning almost every single H atom leaves its "X" and becomes an H+ ion. Examples of strong acids include hydrochloric acid (HCl – the stuff in your stomach), sulfuric acid (H2SO4 – used in car batteries), and nitric acid (HNO3 – used in fertilizers).
Imagine a bowl of Skittles. If you pour water into the bowl and every single Skittle instantly dissolves, that's a strong acid in action! All the molecules are breaking down and releasing their "flavor" (or in the acid's case, H+ ions) into the solution.
Weak acids, on the other hand, are more hesitant. They only partially dissociate in water. Some H atoms leave their "X" partners, but most stay put. Think of it like a reluctant dance partner – they might agree to a slow dance, but they're not exactly cutting loose on the dance floor. Examples of weak acids include acetic acid (CH3COOH – the acid in vinegar), citric acid (C6H8O7 – found in citrus fruits), and carbonic acid (H2CO3 – the fizz in soda).
Going back to the Skittles analogy, imagine pouring water on a different kind of candy that only dissolves a little bit. You might get some color in the water, but the candy itself mostly stays intact. That's how weak acids behave in water. They release some H+ ions, but a lot of the acid molecules stay together.
pH: Measuring the Acidic Vibe
So, how do we measure how acidic something is? That's where pH comes in. pH is like a report card for acidity. It's a scale that runs from 0 to 14, with 7 being neutral (like pure water).
A pH below 7 indicates an acidic solution. The lower the pH, the more acidic it is, and the more H+ ions are floating around. A pH of 1 is super acidic (think battery acid!), while a pH of 6 is only slightly acidic (like coffee).
A pH above 7 indicates a basic or alkaline solution (the opposite of acidic). These solutions have fewer H+ ions and more hydroxide ions (OH-). Things like bleach and baking soda are basic.
You can think of pH like the volume knob on your stereo. Turning it down low means less sound (less H+ ions), and turning it up high means more sound (more H+ ions). The pH scale gives us a standardized way to describe the "volume" of acidity.
Everyday Acids: A World of Sour Surprises
Acids are everywhere in our daily lives, often hiding in plain sight. Here are a few examples, and why they're useful (or sometimes just annoying):
- Citric acid: Found in citrus fruits like lemons and oranges, citric acid gives them their characteristic sour taste. It's also used as a flavoring and preservative in many foods and drinks. Think of it as the zesty friend who always brightens up the party (or your morning juice).
- Acetic acid: This is the main component of vinegar, which is used for everything from salad dressings to cleaning to pickling. It's the reason why vinegar smells so strong and has that distinctive tangy taste.
- Hydrochloric acid: This strong acid is naturally produced in your stomach to help digest food. Sometimes, if you eat too much or too quickly, the acid can reflux up into your esophagus, causing heartburn. That burning sensation is your stomach acid telling you to slow down!
- Lactic acid: Your muscles produce lactic acid during intense exercise. This is what causes that burning feeling you get when you push yourself too hard. It's like your muscles are sending you a text message saying, "Dude, chill out!"
- Carbonic acid: This weak acid is formed when carbon dioxide dissolves in water. It's what gives soda its fizz. Over time, carbonic acid can slowly dissolve rocks, which is why rainwater can erode mountains over millions of years. It's a slow and steady acid that gets the job done!
We use acids in everything, from preserving food to cleaning our homes. Acids are also vital to many industrial processes. Sulfuric acid is the most produced industrial chemical in the world! It's used in the manufacturing of fertilizers, detergents, plastics, and many other products.
Safety First: Handle with Care
While many acids are harmless (and even delicious!), some can be dangerous if not handled properly. Strong acids can cause burns to the skin and eyes, and can even damage materials like metal and fabric. That's why it's important to always wear appropriate safety gear (like gloves and goggles) when working with concentrated acids. Always read the label and follow the instructions carefully.
Think of it like handling fire. You know fire can be helpful (cooking, warmth), but it can also be dangerous if you're not careful. The same goes for acids. Respect their power, and you'll be just fine.
In conclusion, the relationship between acids and water is a fundamental concept in chemistry. It's the key to understanding everything from the taste of your lemonade to the power of your stomach acid. So next time you squeeze a lemon or use vinegar in a recipe, remember the drama queens of the chemistry world and the hydrogen ions they so generously release!
Hopefully, this article has made the magic of acids a little less mysterious and a lot more relatable. Now go forth and impress your friends with your newfound knowledge of H+ ions!
+when+dissolved+in+water+are+called+acids..jpg)





+and+anions.jpg)


+when+dissolved+in+water..jpg)






.jpg)
+when+dissolved+in+water..jpg)





