Ever tried starting a campfire and ended up with more smoke than flames? Yeah, we've all been there. That struggle, that initial effort needed to get things going, is a lot like activation energy in the world of chemistry. It’s the *oof* you need to overcome before the party really starts – before the chemical reaction kicks into high gear.
Think of it this way: imagine you're trying to push a massive boulder up a hill. The hill represents the activation energy. You need a certain amount of *oomph* to get that boulder to the very top. Once it's over the crest, it'll roll down the other side all on its own – that’s the reaction happening! But you gotta get it over that hill first.
What Exactly IS Activation Energy?
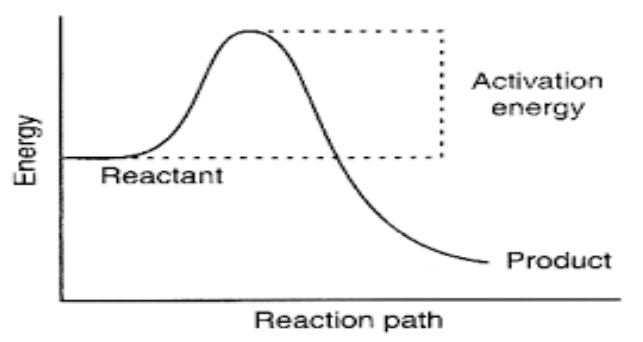
Okay, let’s get a little more *technical* (but not too technical, promise!). Activation energy, often written as Ea, is the minimum amount of energy that reacting species (atoms, molecules, ions – the whole crew) must possess in order to undergo a specified reaction. It’s like the entry fee to the chemical reaction concert.
Basically, for a reaction to happen, the reactants (the ingredients) need to bump into each other, right? But just bumping isn't enough. They need to bump with enough force and in the right orientation. Activation energy provides that force. If they don’t have enough energy, they’ll just bounce off each other like bumper cars with toddlers at the wheel. No reaction. Sad face.
Imagine you're trying to convince your friend to go out on a Friday night. Your friend is the reactant, and going out is the reaction. If you just casually suggest it, they might brush you off. That’s low energy. But if you really hype it up, promise them their favorite pizza, and remind them about that cute person they’ve been wanting to see – you've provided the *activation energy*! Boom, they're in!
So, Which of These Statements About Activation Energy is True?
Now for the main event! Let’s look at some common statements about activation energy and see which ones ring true and which ones are... well, let’s just say *slightly exaggerated*.
Is Activation Energy Always Required?
TRUE-ISH! Most reactions do require activation energy. But there are some rare exceptions. Some reactions are so inherently favorable that they practically happen spontaneously. Think of it like a perfectly balanced house of cards collapsing with the slightest breath – it doesn't need a big shove, just a tiny nudge. These are few and far between, though. Most of the time, you're gonna need that energy boost.
Consider lighting a match. You have to strike it (provide activation energy) to start the combustion reaction. But once the flame is going, it keeps itself going! Initially, you need that little push to get the chemical reaction started.
Does Activation Energy Determine the Reaction Rate?
DEFINITELY TRUE! This is a biggie. The higher the activation energy, the slower the reaction rate. It’s like trying to run a marathon wearing lead boots. It's possible, but it's gonna take a while. Conversely, the lower the activation energy, the faster the reaction rate. Think running a marathon on a downhill slope. Much easier, right?
Think about baking a cake. If you skip preheating the oven (low activation energy), the cake will take forever to bake, and it might not even rise properly. But if you preheat the oven to the right temperature (providing the necessary activation energy), the cake bakes beautifully and quickly.
Chemists use something called the Arrhenius equation to mathematically describe the relationship between activation energy and reaction rate. It's a bit intimidating to look at, but it basically says that as activation energy increases, the rate constant (and therefore the reaction rate) decreases exponentially. So, a small change in activation energy can have a *huge* impact on how fast the reaction goes.
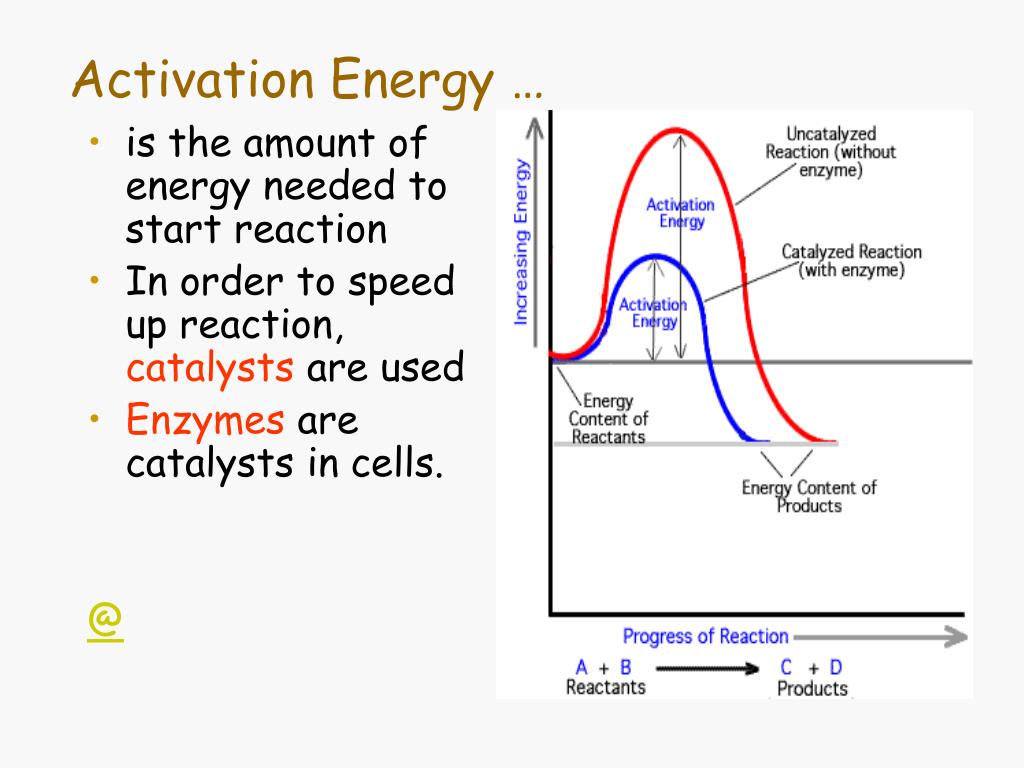
Can Catalysts Lower Activation Energy?
ABSOLUTELY TRUE! This is where things get really interesting. Catalysts are like chemical matchmakers. They speed up reactions without being consumed in the process. They do this by providing an alternative reaction pathway with a lower activation energy.
Imagine you're hiking to the top of a mountain. The original path is steep and difficult (high activation energy). But a catalyst is like a secret, winding trail that's much gentler (lower activation energy). You still reach the same destination, but you get there much faster and with less effort.
Enzymes are biological catalysts that do this magic in our bodies. They allow reactions to occur at body temperature that would otherwise require extreme heat or pressure. Without enzymes, we'd be a very sluggish, uncooked bunch!
For example, the decomposition of hydrogen peroxide (H2O2) into water and oxygen is a slow process on its own. But if you add a catalyst like manganese dioxide (MnO2), the reaction speeds up dramatically. The MnO2 provides a different reaction pathway with a lower activation energy.
Is Activation Energy Always a Positive Value?
MOSTLY TRUE! Activation energy is almost always a positive value. This makes sense because you generally need to *add* energy to get a reaction started. However, there are some extremely rare cases where the activation energy can be zero or even negative. These reactions are usually incredibly fast and spontaneous.
Think of it like this: imagine you're trying to start a domino effect. You need to push the first domino (provide activation energy). That is a positive value. However if the dominoes were already swaying slightly, they’d trigger each other with even less effort, approaching zero. Now imagine instead of dominoes, you lined up unstable, top-heavy cups. They're ready to topple over at the slightest vibration. Once one falls, the resulting shockwave causes the rest to fall much more readily. This is similar to having negative activation energy. That is more theoretical and less common.
For all intents and purposes, especially in introductory chemistry, you can safely assume that activation energy is a positive value.
Does Activation Energy Change With Temperature?
GENERALLY FALSE! The activation energy is usually considered a constant value for a given reaction under specific conditions. However, there can be very slight variations in activation energy with temperature, especially over a very wide temperature range. But for most practical purposes, we treat it as a constant.
It's like saying the height of a mountain doesn't change with the weather. While the amount of snow on the peak might vary with the season, the mountain's actual height remains the same.
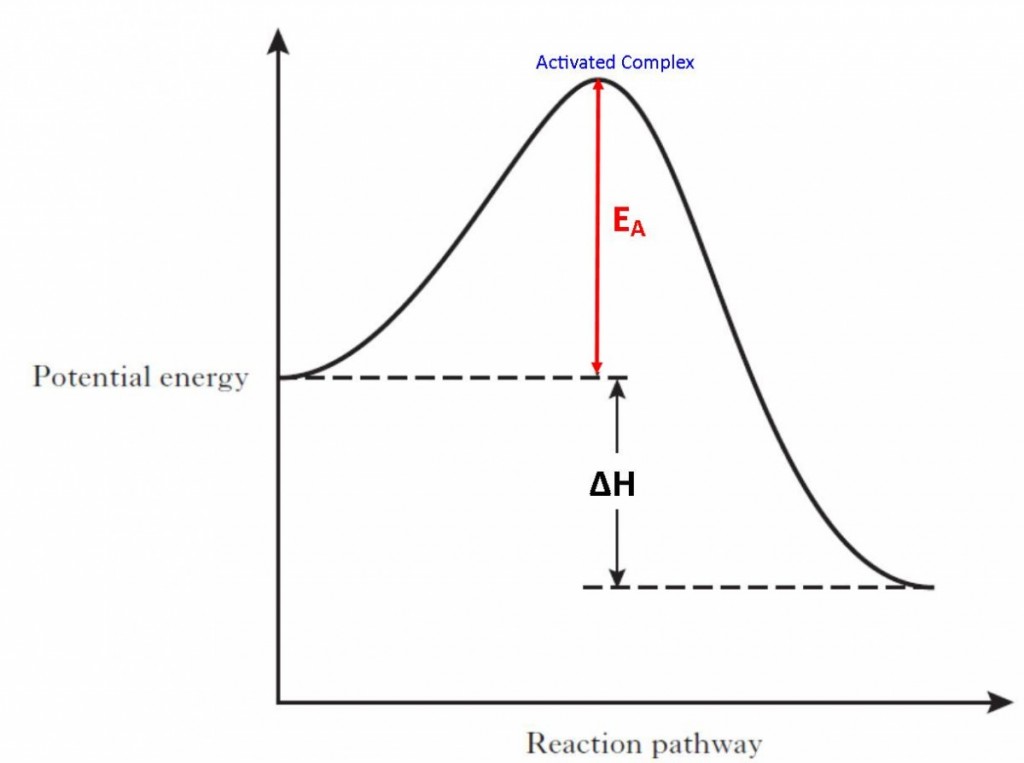
Is Activation Energy the Same as the Change in Enthalpy (ΔH)?
DEFINITELY FALSE! This is a common point of confusion. Activation energy (Ea) is the energy required to reach the transition state (the top of that hill we talked about earlier). Change in enthalpy (ΔH) is the difference in energy between the reactants and the products. They're related, but they're not the same thing.
Think of it like this: imagine you're driving from point A to point B. The activation energy is the amount of gas you need to get over the highest hill on the way. The change in enthalpy is the difference in altitude between point A and point B. You might have to climb a big hill to get there (high activation energy), but if point B is lower than point A (negative ΔH, exothermic reaction), you'll end up with less potential energy than you started with. Or vice versa.
For an *exothermic* reaction (releases heat), ΔH is negative. For an *endothermic* reaction (absorbs heat), ΔH is positive. The activation energy, however, is always positive (or very close to it) regardless of whether the reaction is exothermic or endothermic.
The Takeaway
So, there you have it! Activation energy is the *hurdle* that reactants need to clear to become products. It determines the reaction rate, can be lowered by catalysts, and is generally a positive value. Understanding activation energy is key to understanding how chemical reactions work and how we can control them. And hey, maybe it'll even help you start a better campfire next time!
Remember, chemistry isn't just about equations and formulas; it's about understanding the world around us, one activation energy at a time.





.jpg)
.jpg)