Ever wonder why your phone doesn't give you a little electric shock every time you touch it? Or why your favorite sweater doesn’t suddenly cling to you with the force of a thousand tiny magnets? A big part of the reason is because of something incredibly small: atoms. And specifically, the fact that they have no overall charge. Sounds a bit sci-fi, right? But trust me, it's easier than you think to understand, and it's kind of a big deal for, well, everything!
The Balancing Act: Positive vs. Negative
Think of an atom like a tiny, bustling city. In the center, we have the nucleus, the city hall, containing protons and neutrons. Protons are like the city's enthusiastic cheerleaders – always positive and full of energy (hence, they have a positive charge!). Neutrons, on the other hand, are like the calm, neutral librarians, just quietly doing their job with no charge at all.
Now, circling around the nucleus, like speedy little race cars on a track, are electrons. These guys are negatively charged. They’re like the city's rebels, always doing the opposite of the protons.
So, what makes an atom neutral? It's all about balance! Imagine a seesaw. On one side, you have the positive protons in the nucleus. On the other side, you have the negative electrons zooming around. For an atom to be neutral, the number of protons (positive charges) must exactly equal the number of electrons (negative charges). It's a perfect, cosmic balancing act!
If you have, say, 6 protons, you need 6 electrons to cancel out the charges and make the whole atom neutral. Too many protons and the atom becomes positively charged; too many electrons and it becomes negatively charged. These charged atoms are called ions, and while they are very important, most of the stuff around you is made of neutral atoms.
A Simple Analogy: The Magnet Game
Let's play a quick game with magnets. Imagine you have a stack of "positive" magnets and a stack of "negative" magnets. If you put one positive and one negative magnet together, they cancel each other out, right? They stick together, but the overall magnetic effect disappears. That's exactly what happens inside an atom. Each positive proton cancels out the charge of a negative electron, resulting in a neutral overall charge.
Now, imagine you have three positive magnets and only one negative magnet. You'll still have some magnetic effect left – a positive one. That's like an atom becoming an ion with a positive charge.
Why Should You Care? (Besides Avoiding Shocks!)
Okay, so atoms are neutral. Big deal, right? Actually, it's a huge deal! Here's why:
* The World Around You: Imagine if atoms weren't neutral. Everything would be clinging together or repelling each other like crazy! Your chair might try to fling you across the room, or your clothes might refuse to touch your skin. The fact that atoms are generally neutral allows them to form stable molecules and materials. The coffee cup sitting on your desk? The paper you’re reading? The air you're breathing? All made possible by the neutrality of atoms! * Chemical Reactions: While neutral atoms are stable, they can also *share* or *transfer* electrons with each other to form molecules. This is what makes chemistry possible! These interactions happen because atoms "want" to achieve a stable electron configuration, and sometimes that means gaining or losing an electron (becoming an ion!) to bond with other atoms. So, even though individual atoms often strive for neutrality, their willingness to become ions allows for the creation of incredible new substances. Think of it as atoms momentarily becoming un-neutral for the greater good of forming a molecule. * Electricity: Electricity, at its core, is the flow of electrons. And while atoms themselves are normally neutral, those electrons can be coaxed into moving from one atom to another. This is what powers your phone, your lights, and pretty much everything else that plugs into a wall. The fact that atoms can sometimes lose or gain electrons (becoming ions) is essential for electrical conductivity. * Life Itself: From the DNA that makes you, you, to the proteins that help your body function, everything about life relies on the specific ways atoms interact with each other. And those interactions are governed by the electrical forces between atoms and molecules. The neutrality of atoms, and their capacity to become ions, is fundamental to all biological processes.The Takeaway: A Cosmic Balancing Act
So, the next time you're enjoying a perfectly normal, non-shocking day, take a moment to appreciate the amazing balancing act happening inside every atom. It's a testament to the elegant simplicity and incredible complexity of the universe. The fact that atoms are neutral is not just a fun fact to know; it's a fundamental principle that underpins the very fabric of reality. From the smallest grain of sand to the largest star, everything is held together (or kept apart!) by the power of the neutral atom.
Think of it like this: the universe is a giant, incredibly complex Lego set. Atoms are the basic Lego bricks, and their neutrality allows them to connect and interact in predictable and stable ways. Without that neutrality, the whole Lego set would collapse into a chaotic mess!
It's pretty cool when you think about it, right? Something so small can have such a profound impact on everything around us. So next time you’re bored, remember the amazing, charge-balanced atom and give it a little mental high-five!

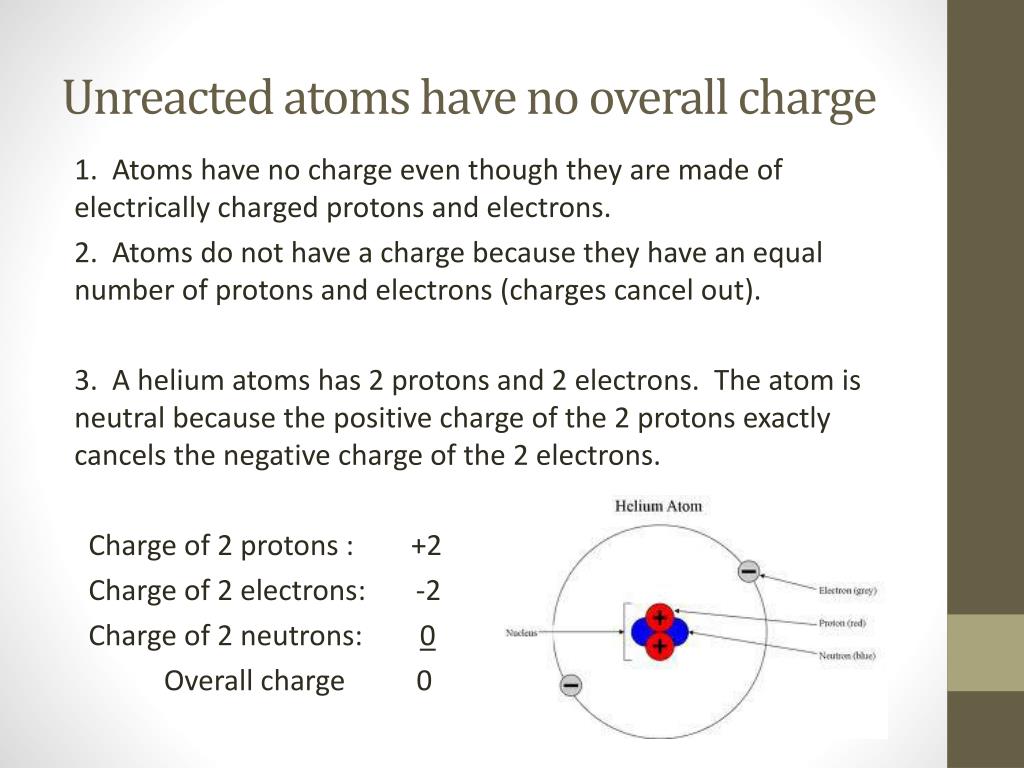



+and+electrons+(negative+charge)+and+so+have+no+overall+charge.jpg)
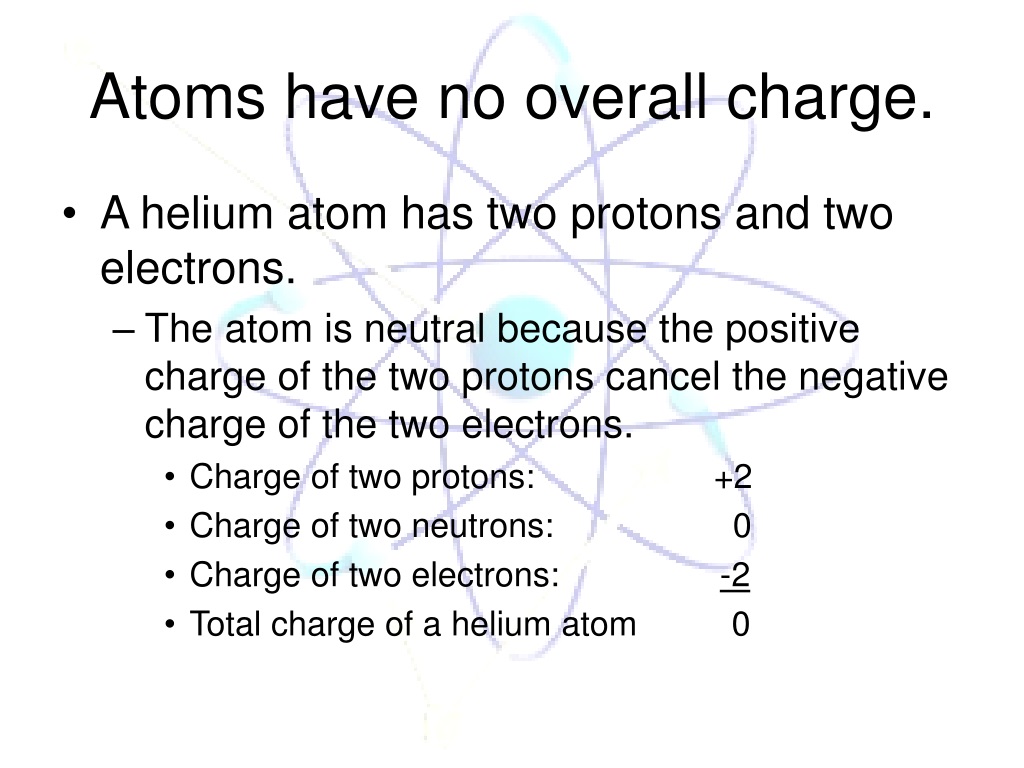



.jpg)



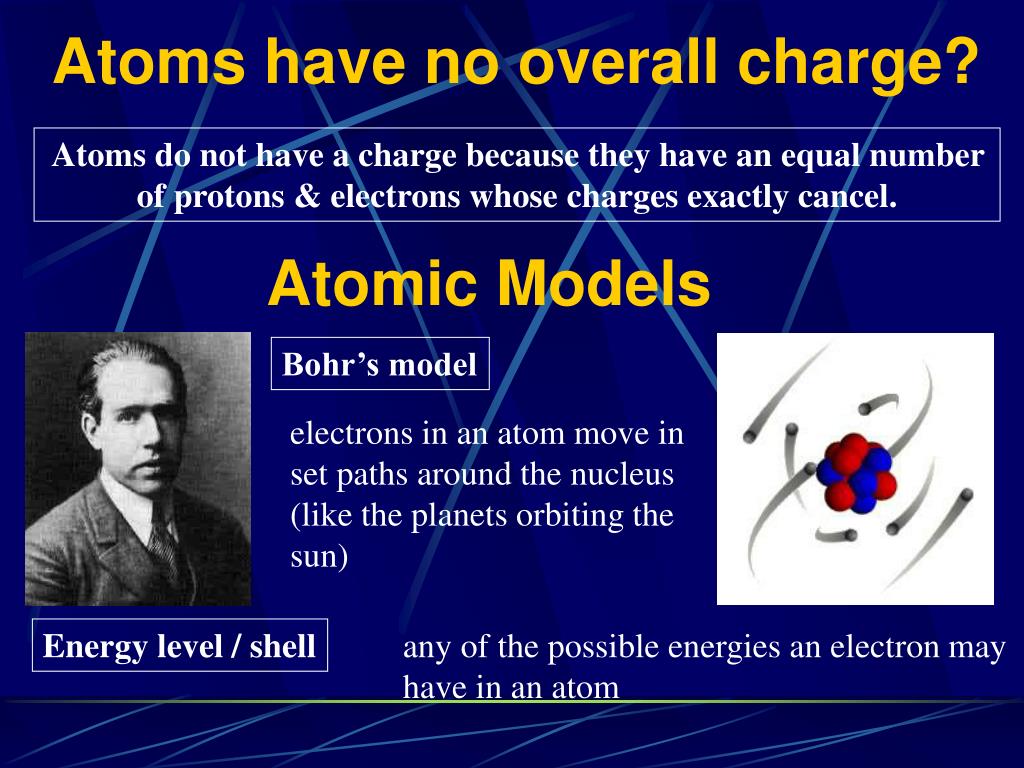


+cancel+out+the+electrons+(-).+Helium+2+protons.jpg)







